A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
Methods
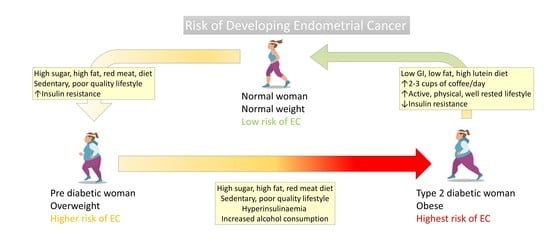
2. Endometrial Cancer Incidence and Prevalence
3. Types of Endometrial Cancer
4. Mechanisms of Oestrogen-Induced Endometrial Cancer
5. Mechanism of Oestrogen-Independent Endometrial Cancer
6. How Metformin and Progestin Protects against EC
7. Dietary Factors
Lipids
8. Vitamins and Minerals
8.1. Vitamin A and Carotenoids
8.2. Vitamin C
8.3. Vitamin E
8.4. Selenium
8.5. Calcium
8.6. Cadmium
9. Plant Derivatives and Hormones
9.1. Lignans
9.2. Soy Isoflavones
9.3. Coffee and Chlorogenic Acid
9.4. Green Tea and (−)-Epigallocatechin-3-Gallate
9.5. Agaricus Mushroom
9.6. Resveratrol
9.7. Curcumin
9.8. Indole-3-Carbinol and Di-Indoylmethane
9.9. Melatonin
10. Clinical Trials and Studies
11. Current Research Models and Future Research Directions
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Powers, S.E.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nat. Cell Biol. 2015, 529, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Trovato, G.M. Behavior, nutrition and lifestyle in a comprehensive health and disease paradigm: Skills and knowledge for a predictive, preventive and personalized medicine. EPMA J. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Smith, M.; Lutgendorf, S.K.; Sood, A.K. Impact of stress on cancer metastasis. Futur. Oncol. 2010, 6, 1863–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Cancer Research Fund. Diet, Nutrition, Physical Activity and Endometrial Cancer. Available online: https://www.wcrf.org/dietandcancer (accessed on 23 January 2021).
- Kruk, J. Self-reported psychological stress and the risk of breast cancer: A case-control study. Stress 2011, 15, 162–171. [Google Scholar] [CrossRef]
- Mouchacca, J.; Abbott, G.R.; Ball, K. Associations between psychological stress, eating, physical activity, sedentary behaviours and body weight among women: A longitudinal study. BMC Public Health 2013, 13, 828. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, N.R.; Strandberg-Larsen, K.; Grønbæk, M.; Kristensen, T.S.; Schnohr, P.; Zhang, Z.-F. Self-reported stress and risk of endometrial cancer: A prospective cohort study. Psychosom. Med. 2007, 69, 383–389. [Google Scholar] [CrossRef]
- Cho, H.J.; Kwon, G.T.; Park, H.; Song, H.; Lee, K.W.; Kim, J.-I.; Park, J.H.Y. A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: Possible contribution of adipose tissue-derived cytokines. Nutrition 2015, 7, 2539–2561. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Aupperlee, M.D.; Zhao, Y.; Tan, Y.S.; Kirk, E.L.; Sun, X.; Troester, M.A.; Schwartz, R.C.; Haslam, S.Z. Pubertal and adult windows of susceptibility to a high animal fat diet in Trp53-null mammary tumorigenesis. Oncotarget 2016, 7, 83409–83423. [Google Scholar] [CrossRef] [Green Version]
- Lee, J. The obesity pandemic and the search for solutions. J. Med. Food 2020, 23, 205. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Shen, J.; Gao, L.; Feng, Y. Estrogen promotes fat mass and obesity-associated protein nuclear localization and enhances endometrial cancer cell proliferation via the mTOR signaling pathway. Oncol. Rep. 2016, 35, 2391–2397. [Google Scholar] [CrossRef] [Green Version]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; López-Vilaró, L.; Nasarre, L.; Perez-Olabarria, M.; Vázquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortés, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pyragius, C.E.; Fuller, M.; Ricciardelli, C.; Oehler, M.K. Aberrant lipid metabolism: An emerging diagnostic and therapeutic target in ovarian cancer. Int. J. Mol. Sci. 2013, 14, 7742–7756. [Google Scholar] [CrossRef] [Green Version]
- Bosetti, C.; Bravi, F.; Turati, F.; Edefonti, V.; Polesel, J.; Decarli, A.; Negri, E.; Talamini, R.; Franceschi, S.; La Vecchia, C.; et al. Nutrient-based dietary patterns and pancreatic cancer risk. Ann. Epidemiol. 2013, 23, 124–128. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, J. Dietary factors and the risk of thyroid cancer: A review. Clin. Nutr. Res. 2014, 3, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Bella, F.; Torrisi, A.; Sciacca, S.; Galvano, F.; Grosso, G. Dietary patterns and risk of colorectal adenoma: A systematic review and meta-analysis of observational studies. J. Hum. Nutr. Diet. 2016, 29, 757–767. [Google Scholar] [CrossRef]
- Magalhães, B.; Peleteiro, B.; Lunet, N. Dietary patterns and colorectal cancer: Systemic review and meta-analysis. Eur. J. Cancer Prev. 2012, 21, 15–23. [Google Scholar] [CrossRef]
- Markaki, I.; Linos, D.; Linos, A. The influence of dietary patterns on the development of thyroid cancer. Eur. J. Cancer 2003, 39, 1912–1919. [Google Scholar] [CrossRef]
- Pericleous, M.; Rossi, R.E.; Mandair, D.; Whyand, T.; Caplin, M.E. Nutrition and pancreatic cancer. Anticancer Res. 2014, 34, 9–21. [Google Scholar] [PubMed]
- Rossi, R.E.; Pericleous, M.; Mandair, D.; Whyand, T.; Caplin, M.E. The role of dietary factors in prevention and progression of breast cancer. Anticancer Res. 2014, 34, 6861–6875. [Google Scholar] [PubMed]
- Malik, T.Y.; Chishti, U.; Aziz, A.B.; Sheikh, I. Comparison of risk factors and survival of Type 1 and Type II endometrial cancers. Pak. J. Med. Sci. 1969, 32, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Uterine Cancer Statistics. 2014. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer (accessed on 25 August 2020).
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Carlson, M.J.; Thiel, K.W.; Yang, S.; Leslie, K.K. Catch it before it kills: Progesterone, obesity, and the prevention of endometrial cancer. Discov. Med. 2012, 14, 215–222. [Google Scholar] [PubMed]
- Lee, W.-L.; Lee, F.-K.; Su, W.-H.; Tsui, K.-H.; Kuo, C.-D.; Hsieh, S.-L.E.; Wang, P.-H. Hormone therapy for younger patients with endometrial cancer. Taiwan. J. Obstet. Gynecol. 2012, 51, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beral, V.; Bull, D.; Reeves, G.; Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet 2005, 365, 1543–1551. [Google Scholar] [CrossRef]
- Berstein, L.; Tsyrlina, E.; Poroshina, T.; Bychkova, N.; Kalinina, N.; Gamajunova, V.; Vasilyev, D.; Kovalenko, I. Switching (overtargeting) of estrogen effects and its potential role in hormonal carcinogenesis. Neoplasma 2002, 49, 21–25. [Google Scholar] [PubMed]
- Woodruff, J.; Pickar, J.H. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. Am. J. Obstet. Gynecol. 1994, 170, 1213–1223. [Google Scholar] [CrossRef]
- Fader, A.N.; Arriba, L.N.; Frasure, H.E.; Von Gruenigen, V.E. Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecol. Oncol. 2009, 114, 121–127. [Google Scholar] [CrossRef]
- Friberg, E.; Wallin, A.; Wolk, A. Sucrose, high-sugar foods, and risk of endometrial cancer—A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.T.; Hankin, J.H.; Wilkens, L.R.; Lyu, L.C.; McDuffie, K.; Liu, L.Q.; Kolonel, L.N. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997, 57, 5077–5085. [Google Scholar] [PubMed]
- Hu, F.B. Overweight and obesity in women: Health risks and consequences. J. Women’s Health 2003, 12, 163–172. [Google Scholar] [CrossRef]
- McTiernan, A.; Irwin, M.; Vongruenigen, V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J. Clin. Oncol. 2010, 28, 4074–4080. [Google Scholar] [CrossRef]
- Nakamura, K.; Hongo, A.; Kodama, J.; Hiramatsu, Y. Fat accumulation in adipose tissues as a risk factor for the development of endometrial cancer. Oncol. Rep. 2011, 26, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Biel, R.K.; Csizmadi, I.; Cook, L.S.; Courneya, K.S.; Magliocco, A.M.; Friedenreich, C.M. Risk of endometrial cancer in relation to individual nutrients from diet and supplements. Public Health Nutr. 2011, 14, 1948–1960. [Google Scholar] [CrossRef] [Green Version]
- Biel, R.K.; Friedenreich, C.M.; Csizmadi, I.; Robson, P.J.; McLaren, L.; Faris, P.; Courneya, K.S.; Magliocco, A.M.; Cook, L.S. Case-Control study of dietary patterns and endometrial cancer risk. Nutr. Cancer 2011, 63, 673–686. [Google Scholar] [CrossRef]
- Hecht, J.L.; Mutter, G.L. Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol. 2006, 24, 4783–4791. [Google Scholar] [CrossRef]
- Tashiro, H.; Blazes, M.S.; Wu, R.; Cho, K.R.; Bose, S.; Wang, S.I.; Li, J.; Parsons, R.; Ellenson, L.H. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997, 57, 3935–3940. [Google Scholar]
- World Health Organization. GLOBOCAN–CANCER TODAY. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/24-Corpus-uteri-fact-sheet.pdf (accessed on 19 April 2021).
- World Health Organization. International Agency for Research on Cancer Today. Available online: https://www.iarc.who.int/infographics/globocan-2018-latest-global-cancer-data/ (accessed on 19 April 2021).
- Zhang, S.; Gong, T.-T.; Liu, F.-H.; Jiang, Y.-T.; Sun, H.; Ma, X.-X.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national burden of endometrial cancer, 1990–2017: Results from the Global Burden of Disease Study, 2017. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J. The effects of diet on circulating sex hormone levels in men. Nutr. Res. Rev. 2000, 13, 159–184. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Hughes, M.C.; Ibiebele, T.I.; Mulligan, A.A.; Kuhnle, G.G.C.; Webb, P.M. Estimated intake of dietary phyto-oestrogens in Australian women and evaluation of correlates of phyto-oestrogen intake. J. Nutr. Sci. 2012, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Bogess, J.F.; Kilgore, J.E.; Tran, A.-Q. Uterine cancer. In Abeloff’s Clinical Oncology, 6th ed.; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier, Inc.: Philadelphia, PA, USA, 2020; pp. 1508–1524. [Google Scholar]
- White, M. Food access and obesity. Obes. Rev. 2007, 8, 99–107. [Google Scholar] [CrossRef]
- Morris, M.J.; Beilharz, J.E.; Maniam, J.; Reichelt, A.C.; Westbrook, R.F. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 2015, 58, 36–45. [Google Scholar] [CrossRef]
- Agarwal, V.R.; Ashanullah, C.I.; Simpson, E.R.; Bulun, S.E. Alternatively spliced transcripts of the aromatase cytochrome P450 (CYP19) gene in adipose tissue of women. J. Clin. Endocrinol. Metab. 1997, 82, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Simpson, E.R. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J. Clin. Endocrinol. Metab. 1994, 78, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Hemsell, D.L.; Grodin, J.M.; Brenner, P.F.; Siiteri, P.K.; Macdonald, P.C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J. Clin. Endocrinol. Metab. 1974, 38, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Lukanova, A.; Lundin, E.; Micheli, A.; Arslan, A.; Ferrari, P.; Rinaldi, S.; Krogh, V.; Lenner, P.; Shore, R.E.; Biessy, C.; et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int. J. Cancer 2003, 108, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomark. Prev. 2002, 11, PMID:12496040. [Google Scholar] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [Green Version]
- Schmandt, R.E.; Iglesias, D.A.; Na Co, N.; Lu, K.H. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am. J. Obstet. Gynecol. 2011, 205, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Tempfer, C.; Hilal, Z.; Kern, P.; Juhasz-Boess, I.; Rezniczek, G. Menopausal hormone therapy and risk of endometrial cancer: A systematic review. Cancers 2020, 12, 2195. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Zwahlen, M.; Kitchener, H.C.; Egger, M.; Renehan, A.G. Body mass index, hormone replacement therapy, and endometrial cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3119–3130. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-C.; Lacey, J.V.; Brinton, L.A.; Hartge, P.; Adams, K.; Mouw, T.; Carroll, L.; Hollenbeck, A.; Schatzkin, A.; Leitzmann, M.F. Lifetime weight historyand endometrialcancerriskby type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Cancer Research UK. Cancer Research Statistics, Cancer Incidence and Mortality in the UK. Available online: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/uterus/ (accessed on 2 September 2020).
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Cannabinoid receptor expression in estrogen-dependent and estrogen-independent endometrial cancer. J. Recept. Signal Transduct. 2018, 38, 385–392. [Google Scholar] [CrossRef]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Howitt, B.E. Molecular pathology. Predict. Progn. Diagn. Markers Uterine Tumors 2016, 9, 405–426. [Google Scholar] [CrossRef]
- Long, B.; Lilyquist, J.; Weaver, A.; Hu, C.; Gnanaolivu, R.; Lee, K.Y.; Hart, S.N.; Polley, E.C.; Bakkum-Gamez, J.N.; Couch, F.J.; et al. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol. Oncol. 2019, 152, 20–25. [Google Scholar] [CrossRef]
- Lax, S.F. Molecular genetic pathways in various types of endometrial carcinoma: From a phenotypical to a molecular-based classification. Virchows Arch. 2004, 444, 213–223. [Google Scholar] [CrossRef]
- Lax, S.F.; Kendall, B.; Tashiro, H.; Slebos, R.J.; Hedrick, L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: Evidence of distinct molecular genetic pathways. Cancer 2000, 88, PMID:10679651. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.; Zheng, W. Insights into endometrial serous carcinogenesis and progression. Int. J. Clin. Exp. Pathol. 2009, 2, 411–432. [Google Scholar] [PubMed]
- Okuda, T.; Sekizawa, A.; Purwosunu, Y.; Nagatsuka, M.; Morioka, M.; Hayashi, M.; Okai, T. Genetics of endometrial cancers. Obstet. Gynecol. Int. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- López-Reig, R.; Fernández-Serra, A.; Romero, I.; Zorrero, C.; Illueca, C.; García-Casado, Z.; Poveda, A.; López-Guerrero, J.A. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al.; The Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Nat. Cell Biol. 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Oberndorfer, F.; Moling, S.; Hagelkruys, L.; Grimm, C.; Polterauer, S.; Sturdza, A.; Aust, S.; Reinthaller, A.; Müllauer, L.; Schwameis, R. Risk reclassification of patients with endometrial cancer based on tumor molecular profiling: First real world data. J. Pers. Med. 2021, 11, 48. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Gotoh, O.; Fukui, N.; Tanaka, N.; Hasumi, K.; Takazawa, Y.; Noda, T.; Mori, S. Two distinct tumorigenic processes in endometrial endometrioid adenocarcinoma. Am. J. Pathol. 2020, 190, 234–251. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Weigelt, B.; Banerjee, S. Molecular targets and targeted therapeutics in endometrial cancer. Curr. Opin. Oncol. 2012, 24, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, H.B.; Haldorsen, I.S.; Trovik, J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012, 13, 353–361. [Google Scholar] [CrossRef]
- Matias-Guiu, X.; Prat, J. Molecular pathology of endometrial carcinoma. Histopathology 2012, 62, 111–123. [Google Scholar] [CrossRef]
- Dedes, K.J.; Wetterskog, D.; Ashworth, A.; Kaye, S.B.; Reis-Filho, J.S. Emerging therapeutic targets in endometrial cancer. Nat. Rev. Clin. Oncol. 2011, 8, 261–271. [Google Scholar] [CrossRef]
- Zhao, S.; Choi, M.; Overton, J.D.; Bellone, S.; Roque, D.M.; Cocco, E.; Guzzo, F.; English, D.P.; Varughese, J.; Gasparrini, S.; et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2916–2921. [Google Scholar] [CrossRef] [Green Version]
- Urick, M.E.; Rudd, M.L.; Godwin, A.K.; Sgroi, D.C.; Merino, M.J.; Bell, D.W. PIK3R1 (p85α) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011, 71, 4061–4067. [Google Scholar] [CrossRef] [Green Version]
- McConechy, M.K.; Ding, J.; Cheang, M.C.; Wiegand, K.C.; Senz, J.; Tone, A.A.; Yang, W.; Prentice, L.M.; Tse, K.; Zeng, T.; et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012, 228, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Le Gallo, M.; N.I.S.C. Program; O’Hara, A.J.; Rudd, M.L.; Urick, M.E.; Hansen, N.F.; O’Neil, N.J.; Price, J.C.; Zhang, S.; England, B.M.; et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat. Genet. 2012, 44, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, E.; Wu, R.-C.; Guan, B.; Wu, G.; Zhang, J.; Wang, Y.; Song, L.; Yuan, X.; Wei, L.; Roden, R.B.; et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J. Natl. Cancer Inst. 2012, 104, 1503–1513. [Google Scholar] [CrossRef]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased incidence of endometrial cancer following the Women’s Health Initiative: An assessment of risk factors. J. Women’s Health 2019, 28, 237–243. [Google Scholar] [CrossRef]
- Key, T.J.; Pike, M.C. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: Its central role in explaining and predicting endometrial cancer risk. Br. J. Cancer 1988, 57, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Barakat, R.R. Benign and hyperplastic endometrial changes associated with tamoxifen use. Oncology 1997, 11. [Google Scholar] [PubMed]
- Dai, Y.; Lin, X.; Xu, W.; Lin, X.; Huang, Q.; Shi, L.; Pan, Y.; Zhang, Y.; Zhu, Y.; Li, C.; et al. MiR-210-3p protects endometriotic cells from oxidative stress-induced cell cycle arrest by targeting BARD1. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002, 21, 7435–7451. [Google Scholar] [CrossRef] [Green Version]
- Jochem, C.; Wallmann-Sperlich, B.; Leitzmann, M.F. The influence of sedentary behavior on cancer risk: Epidemiologic evidence and potential molecular mechanisms. Curr. Nutr. Rep. 2019, 8, 167–174. [Google Scholar] [CrossRef]
- Moore, S.C.; Gierach, G.L.; Schatzkin, A.; Matthews, C.E. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br. J. Cancer 2010, 103, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Blümel, J.E.; Chedraui, P.; Aedo, S.; Fica, J.; Mezones-Holguín, E.; Barón, G.; Bencosme, A.; Benítez, Z.; Bravo, L.M.; Calle, A.; et al. Obesity and its relation to depressive symptoms and sedentary lifestyle in middle-aged women. Maturitas 2015, 80, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Paudyal, P. Epidemiology of endometrial cancer. In Endometrial Cancer: Current Epidemiology, Detection and Management; Farghaly, S.A., Ed.; Nova Biomedical: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Shang, Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat. Rev. Cancer 2006, 6, 360–368. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Neilson, H.K.; Woolcott, C.G.; Wang, Q.; Yasui, Y.; Brant, R.F.; Stanczyk, F.Z.; Campbell, K.L.; Courneya, K.S. Mediators and moderators of the effects of a year-long exercise intervention on endogenous sex hormones in postmenopausal women. Cancer Causes Control. 2011, 22, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2007, 31, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Helmerhorst, H.J.F.; Wijndaele, K.; Brage, S.; Wareham, N.J.; Ekelund, U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes 2009, 58, 1776–1779. [Google Scholar] [CrossRef] [Green Version]
- Cust, A.E.; Kaaks, R.; Friedenreich, C.; Bonnet, F.; Laville, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Jakobsen, M.U.; Chajès, V.; et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2007, 14, 755–767. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Biel, R.K.; Lau, D.C.; Csizmadi, I.; Courneya, K.S.; Magliocco, A.M.; Yasui, Y.; Cook, L.S. Case–control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2384–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, R.S.; Kabat, G.C.; Kim, M.Y.; Wild, R.A.; Shadyab, A.H.; Wactawski-Wende, J.; Ho, G.Y.F.; Reeves, K.W.; Kuller, L.H.; Luo, J.; et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: A prospective study. Cancer Causes Control. 2019, 30, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Mantzoros, C.S.; Wolk, A. Physical activity and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2136–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, N.; Bauman, A.; Brown, W. Too much sitting: A novel and important predictor of chronic disease risk? Br. J. Sports Med. 2008, 43, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Blanck, H.M.; McCullough, M.L.; Patel, A.V.; Gillespie, C.; Calle, E.E.; Cokkinides, V.E.; Galuska, D.A.; Khan, L.K.; Serdula, M.K. Sedentary behavior, recreational physical activity, and 7-Year weight gain among postmenopausal U.S. women. Obesity 2007, 15, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiol. 2013, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, X.; Wang, Y.; Zhang, J.; Lai, R.; Zhang, B.; Zhou, X. The function of uterine UDP-glucuronosyltransferase 1A8 (UGT1A8) and UDP-glucuronosyltransferase 2B7 (UGT2B7) is involved in endometrial cancer based on estrogen metabolism regulation. Hormones 2020, 19, 403–412. [Google Scholar] [CrossRef]
- Behar, J. Physiology and pathophysiology of the biliary tract: The gallbladder and sphincter of Oddi—A review. ISRN Physiol. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile formation and secretion. Compr. Psysiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.A.; Collins, F.; Cousins, F.L.; Zufiaurre, A.E.; Saunders, P.T.K. The impact of 27-hydroxycholesterol on endometrial cancer proliferation. Endocr. Relat. Cancer 2018, 25, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Gong, T.-T.; Li, D.; Wu, Q.-J.; Wang, Y.-Z. Cholesterol consumption and risk of endometrial cancer: A systematic review and dose-response meta-analysis of observational studies. Oncotarget 2016, 7, 16996–17008. [Google Scholar] [CrossRef] [Green Version]
- Lukanova, A.; Zeleniuch-Jacquotte, A.; Lundin, E.; Micheli, A.; Arslan, A.A.; Rinaldi, S.; Muti, P.; Lenner, P.; Koenig, K.L.; Biessy, C.; et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2 and -3 and risk of endometrial cancer. Int. J. Cancer 2003, 108, 262–268. [Google Scholar] [CrossRef]
- Mulholland, H.G.; Murray, L.J.; Cardwell, C.R.; Cantwell, M.M. Dietary glycaemic index, glycaemic load and endometrial and ovarian cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2008, 99, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Nagle, C.M.; Olsen, C.M.; Ibiebele, T.I.; Spurdle, A.B.; Webb, P.M.; the Australian Ovarian Cancer Study Group. Glycemic index, glycemic load and endometrial cancer risk: Results from the Australian National Endometrial Cancer study and an updated systematic review and meta-analysis. Eur. J. Nutr. 2012, 52, 705–715. [Google Scholar] [CrossRef]
- Segawa, T.; Shozu, M.; Murakami, K.; Kasai, T.; Shinohara, K.; Nomura, K.; Ohno, S.; Inoue, M. Aromatase expression in stromal cells of endometrioid endometrial cancer correlates with poor survival. Clin. Cancer Res. 2005, 11, 2188–2194. [Google Scholar] [CrossRef] [Green Version]
- Klopp, A.H.; Zhang, Y.; Solley, T.; Amaya-Manzanares, F.; Marini, F.; Andreeff, M.; Debeb, B.; Woodward, W.; Schmandt, R.; Broaddus, R.; et al. Omental adipose tissue–derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. 2011, 18, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Guidice, L.C. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Practice and Research. Clin. Endocrinol. Metab. 2006, 20, 235–244. [Google Scholar] [CrossRef]
- Navaratnarajah, R.; Pillay, O.C.; Hardiman, P. Polycystic ovary syndrome and endometrial cancer. Semin. Reprod. Med. 2008, 26, 62–71. [Google Scholar] [CrossRef]
- Horn, L.-C.; Meinel, A.; Handzel, R.; Einenkel, J. Histopathology of endometrial hyperplasia and endometrial carcinoma. Ann. Diagn. Pathol. 2007, 11, 297–311. [Google Scholar] [CrossRef]
- Kurman, R.J.; McConnell, T.G. Precursors of endometrial and ovarian carcinoma. Virchowsv Arch. 2009, 456, 1–12. [Google Scholar] [CrossRef]
- Sherman, M.E. Theories of endometrial carcinogenesis: A multidisciplinary approach. Mod. Pathol. 2000, 13, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banno, K.; Kisu, I.; Yanokura, M.; Tsuji, K.; Masuda, K.; Ueki, A.; Kobayashi, Y.; Yamagami, W.; Nomura, H.; Tominaga, E.; et al. Biomarkers in endometrial cancer: Possible clinical applications (Review). Oncol. Lett. 2012, 3, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, E.; Ayhan, A.; Bahadirli-Talbott, A.; Zhao, C.; Shih, I.-M. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am. J. Surg. Pathol. 2014, 38, 660–665. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Spirito, N.; Cionini, L. Clear cell carcinoma of the endometrium: A biological and clinical enigma. Anticancer. Res. 2010, 30, 1327–2334. [Google Scholar] [PubMed]
- Risinger, J.I.; Allard, J.; Chandran, U.; Day, R.; Chandramouli, G.V.R.; Miller, C.; Zahn, C.; Oliver, J.; Litzi, T.; Marcus, C.; et al. Gene expression analysis of early stage endometrial cancers reveals unique transcripts associated with grade and histology but not depth of invasion. Front. Oncol. 2013, 3, 139. [Google Scholar] [CrossRef] [Green Version]
- Lax, S.F.; Pizer, E.S.; Ronnett, B.M.; Kurman, R.J. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum. Pathol. 1998, 29, 551–558. [Google Scholar] [CrossRef]
- Shai, A.; Segev, Y.; Narod, S.A. Genetics of endometrial cancer. Fam. Cancer 2014, 13, 499–505. [Google Scholar] [CrossRef]
- Jia, L.; Liu, Y.; Yi, X.; Miron, A.; Crum, C.P.; Kong, B.; Zheng, W. Endometrial glandular dysplasia with frequent p53 gene mutation: A genetic evidence supporting its precancer nature for endometrial serous carcinoma. Clin. Cancer Res. 2008, 14, 2263–2269. [Google Scholar] [CrossRef] [Green Version]
- Lax, S.F.; Pizer, E.S.; Ronnett, B.M.; Kurman, R.J. Comparison of estrogen and progesterone receptor, Ki-67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum. Pathol. 1998, 29, 924–931. [Google Scholar] [CrossRef]
- Tashiro, H.; Isacson, C.; Levine, R.; Kurman, R.J.; Cho, K.R.; Hedrick, L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am. J. Pathol. 1997, 150, 177–185. [Google Scholar] [PubMed]
- Fadare, O.; Gwin, K.; Desouki, M.M.; Crispens, M.A.; Jones, H.W.; Khabele, D.; Liang, S.X.; Zheng, W.; Mohammed, K.; Hecht, J.L.; et al. The clinicopathologic significance of p53 and BAF-250a (ARID1A) expression in clear cell carcinoma of the endometrium. Mod. Pathol. 2013, 26, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buza, N.; Hui, P. Marked heterogeneity ofHER2/NEUgene amplification in endometrial serous carcinoma. Genes Chromosom. Cancer 2013, 52, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.M.; Guadagni, F.; Marzano, R.; Benevolo, M.; Merola, R.; Giannarelli, D.; Marandino, F.; Vocaturo, G.; Mariani, L.; Mottolese, M. HER-2/neu oncogene amplification and chromosome 17 aneusomy in endometrial carcinoma: Correlation with oncoprotein expression and conventional pathological parameters. J. Exp. Clin. Cancer Res. 2003, 22, 265–271. [Google Scholar] [PubMed]
- Manavi, M.; Bauer, M.; Baghestanian, M.; Berger, A.; Kucera, E.; Pischinger, K.; Battistutti, W.; Czerwenka, K. Oncogenic potential of c-erbB-2 and its association with c-K-ras in premalignant and malignant lesions of the human uterine endometrium. Tumor Biol. 2001, 22, 299–309. [Google Scholar] [CrossRef]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular carcinoma: Etiology and current and future drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liu, J.-Y.; Lin, J.-K.; Chu, J.-S.; Shew, J.-Y. p53 point mutation enhanced by hepatic regeneration in aflatoxin B1-induced rat liver tumors and preneoplastic lesions. Cancer Lett. 1998, 125, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhou, Q.; Gui, S.-Y.; Li, X. The point mutation of p53 gene exon7 in hepatocellular carcinoma from Anhui Province, a non HCC prevalent area in China. World J. Gastroenterol. 2002, 8, 480–482. [Google Scholar] [CrossRef]
- Weng, M.-W.; Lee, H.-W.; Choi, B.; Wang, H.-T.; Hu, Y.; Mehta, M.; Desai, D.; Amin, S.; Zheng, Y.; Tang, M.-S. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249. Oncotarget 2017, 8, 18213–18226. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Byers, L.A.; Kurie, J.M. Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 2014, 12, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Freudenheim, J.L.; Bonner, M.; Krishnan, S.; Ambrosone, C.B.; Graham, S.; McCann, S.E.; Moysich, K.B.; Bowman, E.; Nemoto, T.; Shields, P.G. Diet and alcohol consumption in relation to p53 mutations in breast tumors. Carcinogenesis 2004, 25, 931–939. [Google Scholar] [CrossRef]
- Hill, S.Y.; Rompala, G.; Homanics, G.E.; Zezza, N. Cross-generational effects of alcohol dependence in humans onHRASandTP53methylation in offspring. Epigenomics 2017, 9, 1189–1203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Howard, E.W.; Guo, Z.; Parris, A.B.; Yang, X. p53 pathway determines the cellular response to alcohol-induced DNA damage in MCF-7 breast cancer cells. PLsS ONE 2017, 12, e0175121. [Google Scholar] [CrossRef]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27Kip1 in the cyclin E–cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [Green Version]
- Nahum, A.; Zeller, L.; Danilenko, M.; Prall, O.W.J.; Watts, C.K.W.; Sutherland, R.L.; Levy, J.; Sharoni, Y. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur. J. Nutr. 2006, 45, 275–282. [Google Scholar] [CrossRef]
- Pelucchi, C.; Maso, L.D.; Montella, M.; Parpinel, M.; Negri, E.; Talamini, R.; Giudice, A.; Franceschi, S.; La Vecchia, C. Dietary intake of carotenoids and retinol and endometrial cancer risk in an Italian case–control study. Cancer Causes Control. 2008, 19, 1209–1215. [Google Scholar] [CrossRef]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Zango, G.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. The role of lycopene and its derivatives in the regulation of transcription systems: Implications for cancer prevention. Am. J. Clin. Nutr. 2012, 96, 1173–1178. [Google Scholar] [CrossRef]
- Kavanaugh, C.J.; Trumbo, P.R.; Ellwood, K.C. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 2007, 99, 1074–1085. [Google Scholar] [CrossRef]
- Slattery, M.L.; Curtin, K.; Ma, K.; Edwards, S.; Schaffer, N.; Anderson, K.; Samowitz, W. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol. Biomark. Prev. 2002, 11, 541–548. [Google Scholar] [PubMed]
- Voskuil, D.W.; Kampman, E.; Van Kraats, A.A.; Balder, H.F.; Van Muijen, G.N.; Goldbohm, R.A.; Veer, P.V. p53 over-expression andp53 mutations in colon carcinomas: Relation to dietary risk factors. Int. J. Cancer 1999, 81, 675–681. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Su, P.-Y.; Hao, J.-H.; Sun, Y.-H. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: A meta-analysis of prospective cohort studies. Int. J. Gynecol. Cancer 2013, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.Y.; Park, Y.; Hartge, P.; Hollenbeck, A.R.; Freedman, N.D. The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J. Clin. Endocrinol. Metab. 2013, 98, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berstein, L.M.; Kvatchevskaya, J.O.; Poroshina, T.E.; Kovalenko, I.G.; Tsyrlina, E.V.; Zimarina, T.S.; Ourmantcheeva, A.F.; Ashrafian, L.; Thijssen, J.H.H. Insulin resistance, its consequences for the clinical course of the disease, and possibilities of correction in endometrial cancer. J. Cancer Res. Clin. Oncol. 2004, 130, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Sakoda, L.C.; Frederiksen, K.; Sherman, M.E.; Kjaer, S.K.; Graubard, B.I.; Olsen, J.H.; Mellemkjaer, L. Relationships of uterine and ovarian tumors to pre-existing chronic conditions. Gynecol. Oncol. 2007, 107, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Friberg, E.; Mantzoros, C.S.; Wolk, A. Diabetes and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757. [Google Scholar] [CrossRef]
- Soliman, P.T.; Cui, X.; Zhang, Q.; Hankinson, S.E.; Lu, K.H. Circulating adiponectin levels and risk of endometrial cancer: The prospective Nurses’ Health Study. Am. J. Obstet. Gynecol. 2011, 204, 167.e1–167.e5. [Google Scholar] [CrossRef] [Green Version]
- Soliman, P.T.; Wu, D.; Tortolero-Luna, G.; Schmeler, K.M.; Slomovitz, B.M.; Bray, M.S.; Gershenson, D.M.; Lu, K.H. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer 2006, 106, 2376–2381. [Google Scholar] [CrossRef]
- Cantrell, L.A.; Zhou, C.; Mendivil, A.; Malloy, K.M.; Gehrig, P.A.; Bae-Jump, V.L. Metformin is a potent inhibitor of endometrial cancer cell proliferation—implications for a novel treatment strategy. Gynecol. Oncol. 2010, 116, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Faivre, S.; Kroemer, G.; Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 671–688. [Google Scholar] [CrossRef]
- Soliman, P.T.; Oh, J.C.; Schmeler, K.M.; Sun, C.C.; Slomovitz, B.M.; Gershenson, D.M.; Burke, T.W.; Lu, K.H. Risk factors for young premenopausal women with endometrial cancer. Obstet. Gynecol. 2005, 105, 575–580. [Google Scholar] [CrossRef]
- Galuska, D.; Nolte, L.A.; Zierath, J.R.; Wallberg-Henriksson, H. Effect of metformin on insulin-stimulated glucose transport in isolated skeletal muscle obtained from patients with NIDDM. Diabetologia 1994, 37, 826–832. [Google Scholar] [CrossRef]
- Mu, N.; Wang, Y.; Xue, F. Metformin: A potential novel endometrial cancer therapy. Int. J. Gynecol. Cancer 2012, 22, 181. [Google Scholar] [CrossRef]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [Green Version]
- Jiralerspong, S.; Gonzalez-Angulo, A.M.; Hung, M.-C. Expanding the arsenal: Metformin for the treatment of triple-negative breast cancer? Cell Cycle 2009, 8, 2681–2684. [Google Scholar] [CrossRef] [Green Version]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.-C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Gao, C.; Fang, L.; Zhao, H.-C.; Yao, S.-K. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scand. J. Gastroenterol. 2012, 48, 78–87. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.-L.; Yu, L.; Hu, Q.; Ji, L.; Zhang, Y.; Liao, Q.-P. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2011, 126, 113–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, L.; Sui, L.; Yang, Y.; Liu, X.; Yu, Y.; Zhu, Y.; Feng, Y. Metformin reverses progestin resistance in endometrial cancer cells by downregulating GloI Expression. Int. J. Gynecol. Cancer 2011, 21, 213–221. [Google Scholar] [CrossRef]
- Westin, S.N.; Fellman, B.; Sun, C.C.; Broaddus, R.R.; Woodall, M.L.; Pal, N.; Urbauer, D.L.; Ramondetta, L.M.; Schmeler, K.M.; Soliman, P.T.; et al. Prospective phase II trial of levonorgestrel intrauterine device: Nonsurgical approach for complex atypical hyperplasia and early-stage endometrial cancer. Am. J. Obstet. Gynecol. 2021, 224, 191.e1–191.e15. [Google Scholar] [CrossRef]
- Vereide, A.B.; Kaino, T.; Sager, G.; Arnes, M.; Ørbo, A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-α and ER-β) in human endometrial hyperplasia. Gynecol. Oncol. 2006, 101, 214–223. [Google Scholar] [CrossRef]
- Dai, D.; Wolf, D.M.; Litman, E.S.; White, M.J.; Leslie, K.K. Progesterone inhibits human endometrial cancer cell growth and invasiveness: Down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res. 2002, 62, 881–886. [Google Scholar] [PubMed]
- Montz, F.J.; Bristow, R.E.; Bovicelli, A.; Tomacruz, R.; Kurman, R.J. Intrauterine progesterone treatment of early endometrial cancer. Am. J. Obstet. Gynecol. 2002, 186, 651–657. [Google Scholar] [CrossRef]
- Šmuc, T.; Rižner, T.L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol. Cell Endocrinol. 2009, 301, 74–82. [Google Scholar] [CrossRef]
- Taylor, A.H.; Al-Azzawi, F.; Pringle, J.H.; Bell, S.C. Inhibition of endometrial carcinoma cell growth using antisense estrogen receptor oligodeoxyribonucleotides. Anticancer Res. 2003, 22, 3993–4003. [Google Scholar] [PubMed]
- Lee, L.R.; Teng, P.-N.; Nguyen, H.; Hood, B.L.; Kavandi, L.; Wang, G.; Turbov, J.M.; Thaete, L.G.; Hamilton, C.A.; Maxwell, G.L.; et al. Progesterone enhances calcitriol antitumor activity by upregulating vitamin D receptor expression and promoting apoptosis in endometrial cancer cells. Cancer Prev. Res. 2013, 6, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Ivanova, V.S.; Kavandi, L.; Rodriguez, G.C.; Maxwell, G.L.; Syed, V. Progesterone and 1,25-dihydroxyvitamin D3 inhibit endometrial cancer cell growth by upregulating semaphorin 3B and semaphorin 3F. Mol. Cancer Res. 2011, 9, 1479–1492. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Syed, V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol. Endocrinol. 2010, 27, 830–836. [Google Scholar] [CrossRef]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of vitamin D supplementation by sunlight or oral D3 on exercise performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Fakhoury, H.M.A.; Karras, S.N.; Al Anouti, F.; Bhattoa, H.P. Variations in 25-hydroxyvitamin D in countries from the Middle East and Europe: The roles of UVB exposure and diet. Nutrients 2019, 11, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandera, E.V.; Williams, M.G.; Sima, C.; Bayuga, S.; Pulick, K.; Wilcox, H.; Soslow, R.; Zauber, A.G.; Olson, S.H. Phytoestrogen consumption and endometrial cancer risk: A population-based case–control study in New Jersey. Cancer Causes Control 2009, 20, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, M.T.; Wilkens, L.R.; Hankin, J.H.; Lyu, L.-C.; Wu, A.H.; Kolonel, L.N. Association of soy and fiber consumption with the risk of endometrial cancer. Am. J. Epidemiol. 1997, 146, 294–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genkinger, J.M.; Friberg, E.; Goldbohm, R.A.; Wolk, A. Long-term dietary heme iron and red meat intake in relation to endometrial cancer risk. Am. J. Clin. Nutr. 2012, 96, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallianpur, A.R.; Lee, S.-A.; Xu, W.-H.; Zheng, W.; Gao, Y.-T.; Cai, H.; Ruan, Z.-X.; Xiang, Y.-B.; Shu, X.O. Dietary iron intake and risk of endometrial cancer: A population-based case-control study in Shanghai, China. Nutr. Cancer 2009, 62, 40–50. [Google Scholar] [CrossRef]
- Terry, P.; Vainio, H.; Wolk, A.; Weiderpass, E. Dietary factors in relation to endometrial cancer: A nationwide case-control study in Sweden. Nutr. Cancer 2002, 42, 25–32. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [Green Version]
- Laviano, A.; Rianda, S.; Molfino, A.; Fanelli, F.R. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 156–161. [Google Scholar] [CrossRef]
- Arem, H.; Neuhouser, M.L.; Irwin, M.L.; Cartmel, B.; Lu, L.; Risch, H.; Mayne, S.T.; Yu, H. Omega-3 and omega-6 fatty acid intakes and endometrial cancer risk in a population-based case-control study. Eur. J. Nutr. 2012, 52, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n−3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Long-chain omega-3 fatty acids and cancer. Any cause for concern. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.; Tarín, J.; Cano, A. The Mediterranean diet: A historical perspective on food for health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Altomare, R.; Cacciabaudo, F.; Damiano, G.; Palumbo, V.D.; Gioviale, M.C.; Bellavia, M.; Tomasello, G.; Monte, A.I.L. The Mediterranean diet: A history of health. Iran. J. Public Health 2013, 42, 449–457. [Google Scholar] [PubMed]
- Hadziabdić, M.O.; Bozikov, V.; Pavić, E.; Romić, Z. The antioxidative protecting role of the Mediterranean diet. Coll. Antropol. 2012, 36, 1427–1434. [Google Scholar] [PubMed]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873. [Google Scholar] [PubMed]
- Dalvi, T.B.; Canchola, A.J.; Horn-Ross, P.L. Dietary patterns, Mediterranean diet, and endometrial cancer risk. Cancer Causes Control 2007, 18, 957–966. [Google Scholar] [CrossRef]
- Ricceri, F.; Giraudo, M.T.; Fasanelli, F.; Milanese, D.; Sciannameo, V.; Fiorini, L.; Sacerdote, C. Diet and endometrial cancer: A focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer 2017, 17, 757. [Google Scholar] [CrossRef] [Green Version]
- Ju, W. Red meat intake and the risk of endometrial cancer: Meta-analysis of observational studies. World J. Meta Anal. 2015, 3, 125–132. [Google Scholar] [CrossRef]
- Bandera, E.V.; Kushi, L.H.; Moore, D.F.; Gifkins, D.M.; McCullough, M.L. Consumption of animal foods and endometrial cancer risk: A systematic literature review and meta-analysis. Cancer Causes Control 2007, 18, 967–988. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Kushi, L.H.; Potter, J.D.; Sellers, T.A.; Doyle, T.J.; Bostick, R.M.; Folsom, A.R. Dietary intake of energy and animal foods and endometrial cancer incidence. Am. J. Epidemiol. 1995, 142, 388–394. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef] [Green Version]
- Bingham, S.A. High-meat diets and cancer risk. Proc. Nutr. Soc. 1999, 58, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, T.J.; Appleby, P.N.; Spencer, E.A.; Travis, R.C.; Roddam, A.W.; Allen, N.E. Cancer incidence in vegetarians: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 2009, 89, 1620–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, T.J.; Appleby, P.N.; Spencer, E.A.; Travis, R.C.; Allen, N.E.; Thorogood, M.; Mann, J.I. Cancer incidence in British vegetarians. Br. J. Cancer 2009, 101, 192–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, G.E. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am. J. Clin. Nutr. 1999, 70, 532–538. [Google Scholar] [CrossRef]
- Maximova, K.; Moez, E.K.; Dabravolskaj, J.; Ferdinands, A.R.; Dinu, I.; Siou, G.L.; Al Rajabi, A.; Veugelers, P.J. Co-consumption of vegetables and fruit, whole grains, and fiber reduces the cancer risk of red and processed meat in a large prospective cohort of adults from Alberta’s Tomorrow Project. Nutrients 2020, 12, 2265. [Google Scholar] [CrossRef]
- Zuniga, K.B.; Chan, J.M.; Ryan, C.J.; Kenfield, S.A. Diet and lifestyle considerations for patients with prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 105–117. [Google Scholar] [CrossRef]
- Shi, Z.; Rundle, A.; Genkinger, J.M.; Cheung, Y.K.; Ergas, I.J.; Roh, J.M.; Kushi, L.H.; Kwan, M.L.; Greenlee, H. Distinct trajectories of fruits and vegetables, dietary fat, and alcohol intake following a breast cancer diagnosis: The Pathways Study. Breast Cancer Res. Treat. 2019, 179, 229–240. [Google Scholar] [CrossRef]
- Mossine, V.V.; Mawhinney, T.P.; Giovannucci, E.L. Dried fruit intake and cancer: A systematic review of observational studies. Adv. Nutr. 2019, 11, 237–250. [Google Scholar] [CrossRef]
- Johansson, A.; Acosta, S. Diet and lifestyle as risk factors for carotid artery disease: A prospective cohort study. Cerebrovasc. Dis. 2020, 49, 563–569. [Google Scholar] [CrossRef]
- Thomson, C.A.; Rock, C.L.; Giuliano, A.R.; Newton, T.R.; Cui, H.; Reid, P.M.; Green, T.L.; Alberts, D.S. Longitudinal changes in body weight and body composition among women previously treated for breast cancer consuming a high-vegetable, fruit and fiber, low-fat diet. Eur. J. Nutr. 2004, 44, 18–25. [Google Scholar] [CrossRef]
- Buijsse, B.; Feskens, E.J.M.; Schulze, M.B.; Forouhi, N.G.; Wareham, N.J.; Sharp, S.; Palli, D.; Tognon, G.; Halkjaer, J.; Tjønneland, A.; et al. Fruit and vegetable intakes and subsequent changes in body weight in European populations: Results from the project on Diet, Obesity, and Genes (DiOGenes). Am. J. Clin. Nutr. 2009, 90, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, S.; Chandraratna, R.A. Vitamin A and regulation of gene expression. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 341–346. [Google Scholar] [CrossRef]
- Brtko, J.; Thalhamer, J. Renaissance of the biologically active vitamin A derivatives: Established and novel directed therapies for cancer and chemoprevention. Curr. Pharm. Des. 2003, 9, 2067–2077. [Google Scholar] [CrossRef]
- Disepio, D.; Ghosn, C.; Eckert, R.L.; Deucher, A.; Robinson, N.; Duvic, M.; Chandraratna, R.A.S.; Nagpal, S. Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc. Natl. Acad. Sci. USA 1998, 95, 14811–14815. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-H.; Dai, Q.; Xiang, Y.-B.; Zhao, G.-M.; Ruan, Z.-X.; Cheng, J.-R.; Zheng, W.; Shu, X.O. Nutritional factors in relation to endometrial cancer: A report from a population-based case-control study in Shanghai, China. Int. J. Cancer 2007, 120, 1776–1781. [Google Scholar] [CrossRef] [Green Version]
- Yeh, M.; Moysich, K.B.; Jayaprakash, V.; Rodabaugh, K.J.; Graham, S.; Brasure, J.R.; McCann, S.E. Higher intakes of vegetables and vegetable-related nutrients are associated with lower endometrial cancer risks. J. Nutr. 2008, 139, 317–322. [Google Scholar] [CrossRef]
- Levi, F.; Franceschi, S.; Negri, E.; La Vecchia, C. Dietary factors and the risk of endometrial cancer. Cancer 1993, 71, 3575–3581. [Google Scholar] [CrossRef]
- Kabat, G.C.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Rohan, T.E. Intake of fruits and vegetables, and risk of endometrial cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. 2010, 34, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The carotenoid fucoxanthin can sensitize multidrug resistant cancer cells to doxorubicin via induction of apoptosis, inhibition of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef]
- Veprik, A.; Khanin, M.; Linnewiel-Hermoni, K.; Danilenko, M.; Levy, J.; Sharoni, Y. Polyphenols, isothiocyanates, and carotenoid derivatives enhance estrogenic activity in bone cells but inhibit it in breast cancer cells. Am. J. Physiol. Metab. 2012, 303, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Bandera, E.V.; Gifkins, D.M.; Moore, D.F.; McCullough, M.L.; Kushi, L.H. Antioxidant vitamins and the risk of endometrial cancer: A dose–response meta-analysis. Cancer Causes Control 2009, 20, 699–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper, C.; Molenaar, I.G.M.; Dachs, G.U.; Currie, M.J.; Sykes, P.H.; Vissers, M.C.M. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010, 70, 5749–5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Brasure, J.R.; Swanson, M.K.; Graham, S. Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control 2000, 11, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Bae, S.; Kim, H.; Kim, Y.; Chu, N.B.; Chu, N.K.; Kang, J.S.; Lee, W.J. The anti-tumor activity of vitamin C via the increase of Fas (CD95) and MHC I expression on human stomach cancer cell line, SNU1. Immune Netw. 2011, 11, 210–215. [Google Scholar] [CrossRef]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid); StatPearls Publishing: Treasure Island, FL, USA, 2003; Available online: https://www.statpearls.com/ArticleLibrary/viewarticle/31221 (accessed on 19 April 2021).
- Zhang, D.; Xu, P.; Li, Y.; Wei, B.; Yang, S.; Zheng, Y.; Lyu, L.; Deng, Y.; Zhai, Z.; Li, N.; et al. Association of vitamin C intake with breast cancer risk and mortality: A meta-analysis of observational studies. Aging 2020, 12, 18415–18435. [Google Scholar] [CrossRef]
- Codini, M. Why vitamin C could be an excellent complementary remedy to conventional therapies for breast cancer. Int. J. Mol. Sci. 2020, 21, 8397. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, 8707. [Google Scholar] [CrossRef]
- Lee, A.W.; Wu, A.H.; Wiensch, A.; Mukherjee, B.; Terry, K.L.; Harris, H.R.; Carney, M.E.; Jensen, A.; Cramer, D.W.; Berchuck, A.; et al. Estrogen plus progestin hormone therapy and ovarian cancer. Epidemiology 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Mori, H.; Chen, S. White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft. J. Nutr. Biochem. 2021, 89, 108580. [Google Scholar] [CrossRef]
- Badary, D.M.; Abou-Taleb, H. Vitamin D receptor and cellular retinol-binding protein-1 immunohistochemical expression in normal, hyperplastic and neoplastic endometrium: Possible diagnostic and therapeutic implications. Ann. Diagn. Pathol. 2020, 48, 151569. [Google Scholar] [CrossRef]
- Duman, I.; Tiftik, R.N.; Ün, I. Effects of vitamin D analogs alfacalcidol and calcitriol on cell proliferation and migration of HEC1A endometrial adenocarcinoma cells. Nutr. Cancer 2020, 73, 273–281. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Kojs, Z.; Bednarek, W.; Huczyński, A. Role of vitamin D3 in selected malignant neoplasms. Nutrition 2020, 79–80, 110964. [Google Scholar] [CrossRef]
- Negri, E.; La Vecchia, C.; Franceschi, S.; Levi, F.; Parazzini, F. Intake of selected micronutrients and the risk of endo-metrial carcinoma. Cancer 1996, 77, 917–923. [Google Scholar] [CrossRef]
- Wu, K.; Willett, W.C.; Chan, J.M.; Fuchs, C.S.; Colditz, G.A.; Rimm, E.B.; Giovannucci, E.L. A prospective study on supplemental vitamin E intake and risk of colon cancer in women and men. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1298–1304. [Google Scholar] [PubMed]
- Graham, S.; Zielezny, M.; Marshall, J.; Priore, R.; Freudenheim, J.; Brasure, J.; Haughey, B.; Nasca, P.; Zdeb, M. Diet in the epidemiology of postmenopausal breast cancer in the New York State cohort. Am. J. Epidemiol. 1992, 136, 1327–1337. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Smolarek, A.K.; So, J.Y.; Thomas, P.E.; Lee, H.J.; Paul, S.; Dombrowski, A.; Wang, C.-X.; Saw, C.L.-L.; Khor, T.O.; Kong, A.-N.T.; et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERα, PPARγ, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol. Carcinog. 2012, 52, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Jeong, N.-H.; Song, E.-S.; Lee, J.-M.; Lee, K.-B.; Kim, M.-K.; Cheon, J.-E.; Lee, J.-K.; Son, S.-K.; Lee, J.-P.; Kim, J.-H.; et al. Plasma carotenoids, retinol and tocopherol levels and the risk of ovarian cancer. Acta Obstet. Gynecol. Scand. 2009, 88, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Bandera, C.A.; Magrina, J.F. Robotic surgery in gynecologic oncology. Curr. Opin. Obstet. Gynecol. 2009, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lazaro, C.I.; Martínez-González, M.A.; Aguilera-Buenosvinos, I.; Gea, A.; Ruiz-Canela, M.; Romanos-Nanclares, A.; Toledo, E. Dietary antioxidant vitamins and minerals and breast cancer risk: Prospective results from the SUN cohort. Antioxidants 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Dietary prevention of breast cancer in high-risk women: Role of carotenoids. Am. J. Clin. Nutr. 2021, 113, 499–500. [Google Scholar] [CrossRef]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Franciosi, M.L.M.; Cardoso, A.M. Oxidative damage and antioxidants in cervical cancer. Int. J. Gynecol. Cancer 2020, 31, 265–271. [Google Scholar] [CrossRef]
- Zare, M.; Roshan, Z.N.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2020, 61, 522–534. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol. Trace Element Res. 2003, 94, 113–122. [Google Scholar] [CrossRef]
- Ji, J.; Liu, J.; Liu, H.; Wang, Y. Effects of fermented mushroom of Cordyceps sinensis, rich in selenium, on uterine cervix cancer. Evid. Based Complement. Altern. Med. 2014, 2014, 173180. [Google Scholar] [CrossRef]
- Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Muecke, R. Selenium in oncology: From chemistry to clinics. Molecules 2009, 14, 3975–3988. [Google Scholar] [CrossRef]
- Rudolf, E.; Rudolf, K.; Cervinka, M. Selenium activates p53 and p38 pathways and induces caspase-independent cell death in cervical cancer cells. Cell Biol. Toxicol. 2008, 24, 123–141. [Google Scholar] [CrossRef]
- Shah, Y.M.; Al-Dhaheri, M.; Dong, Y.; Ip, C.; Jones, F.E.; Rowan, B.G. Selenium disrupts estrogen receptor α signaling and potentiates tamoxifen antagonism in endometrial cancer cells and tamoxifen-resistant breast cancer cells. Mol. Cancer Ther. 2005, 4, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Ganash, M. Anticancer potential of ascorbic acid and inorganic selenium on human breast cancer cell line MCF-7 and colon carcinoma HCT-116. J. Cancer Res. Ther. 2021, 17, 122–129. [Google Scholar] [CrossRef]
- Liang, Z.-Z.; Zhu, R.-M.; Li, Y.-L.; Jiang, H.-M.; Li, R.-B.; Wang, Q.; Tang, L.-Y.; Ren, Z.-F. Differential epigenetic profiles induced by sodium selenite in breast cancer cells. J. Trace Elem. Med. Biol. 2021, 64, 126677. [Google Scholar] [CrossRef]
- British Nutrition Foundation. Dietary calcium and health. Available online: https://www.nutrition.org.uk/bnf-publications/briefingpapers.html (accessed on 1 September 2020).
- Bluwstein, A.; Kumar, N.; Leger, K.; Traenkle, J.; Van Oostrum, J.; Rehrauer, H.; Baudis, M.; Hottiger, M.O. PKC signaling prevents irradiation-induced apoptosis of primary human fibroblasts. Cell Death Dis. 2013, 4, e498. [Google Scholar] [CrossRef] [Green Version]
- McCullough, M.L.; Bandera, E.V.; Moore, D.F.; Kushi, L.H. Vitamin D and calcium intake in relation to risk of endometrial cancer: A systematic review of the literature. Prev. Med. 2008, 46, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Martinez, E.; Lazcano-Ponce, E.; Sanchez-Zamorano, L.; Gonzalez-Lira, G.; Rios, P.E.-D.L.; Hernandez-Avila, M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int. J. Gynecol. Cancer 2005, 15, 938–945. [Google Scholar] [CrossRef]
- Youn, J.; Park, S.; Song, S.; Moon, H.; Noh, D.; Jung, S.; Lee, E.; Kim, Z.; Youn, H.J.; Cho, J.; et al. Nutrient intakes from supplement and factors associated with supplement use among breast cancer survivors: A cross-sectional study. Eur. J. Cancer Care 2021, 13447. [Google Scholar] [CrossRef]
- Scherer, G.; Barkemeyer, H. Cadmium concentrations in tobacco and tobacco smoke. Ecotoxicol. Environ. Saf. 1983, 7, 71–78. [Google Scholar] [CrossRef]
- American Toxic Substances and Disease Registry (ATSDR). Toxicological profile for cadmium. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 2 September 2020).
- The International Agency for Research on Cancer (IARC). Tobacco smoking. In Personal Habits and Indoor Combustions; Volume 100E, ISBN 1-3-9789-2832-1373. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Personal-Habits-And-Indoor-Combustions-2012 (accessed on 23 January 2021).
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Cadmium–a metallohormone? Toxicol. Appl. Pharmacol. 2009, 238, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Fontaine, J.-M.; Bartl, I.; Behnam, B.; Welsh, M.J.; Benndorf, R. Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor–positive breast cancer cells. Cell Stress Chaperon. 2007, 12, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Penttinen-Damdimopoulou, P.E.; Mäkelä, S.I.; Berglund, M.; Stenius, U.; Åkesson, A.; Håkansson, H.; Halldin, K. Estrogen-like effects of cadmium in vivo do not appear to be mediated via the classical estrogen receptor transcriptional Ppthway. Environ. Health Perspect. 2010, 118, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Felix, A.S.; Yang, H.P.; Gierach, G.L.; Park, Y.; Brinton, L.A. Cigarette smoking and endometrial carcinoma risk: The role of effect modification and tumor heterogeneity. Cancer Causes Control. 2014, 25, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Al-Zoughool, M.; Dossus, L.; Kaaks, R.; Clavel-Chapelon, F.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; Gauthier, E.; Linseisen, J.; et al. Risk of endometrial cancer in relationship to cigarette smoking: Results from the EPIC study. Int. J. Cancer 2007, 121, 2741–2747. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Feskanich, D.; De Vivo, I.; Hunter, D.J.; Barbieri, R.L.; Rosner, B.; Colditz, G.A.; Hankinson, S.E. Smoking and the risk of endometrial cancer: Results from the Nurses’ Health Study. Int. J. Cancer 2005, 114, 996–1001. [Google Scholar] [CrossRef]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.-I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef]
- Bloomfield, M.; Louie, M.C. Chronic cadmium exposure decreases the dependency of MCF7 breast cancer cells on ERα. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. Anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Jungeström, M.B.; Thompson, L.U.; Dabrosin, C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin. Cancer Res. 2007, 13, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederroth, C.R.; Nef, S. Soy, phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; John, E.M.; Canchola, A.J.; Stewart, S.L.; Lee, M.M. Phytoestrogen intake and endometrial cancer risk. J. Natl. Cancer Inst. 2003, 95, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.K.; Thompson, L.U. Flaxseed and its lignan and oil components: Can they play a role in reducing the risk of and improving the treatment of breast cancer? Appl. Physiol. Nutr. Metab. 2014, 39, 663–678. [Google Scholar] [CrossRef]
- Zaineddin, A.K.; Buck, K.; Vrieling, A.; Heinz, J.; Flesch-Janys, D.; Linseisen, J.; Chang-Claude, J. The association between dietary lignans, phytoestrogen-rich Foods, and fiber intake and postmenopausal breast cancer risk: A German case-control study. Nutr. Cancer 2012, 64, 652–665. [Google Scholar] [CrossRef]
- Gikas, P.D.; Mokbel, K. Phytoestrogens and the risk of breast cancer: A review of the literature. Int. J. Fertil. Women’s Med. 2006, 50, 250–258. [Google Scholar] [PubMed]
- Ollberding, N.J.; Lim, U.; Wilkens, L.R.; Setiawan, V.W.; Shvetsov, Y.B.; Henderson, B.E.; Kolonel, L.N.; Goodman, M.T. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J. Natl. Cancer Inst. 2011, 104, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.H.; Xu, W.H.; Zheng, W.; Gao, Y.T.; Ruan, Z.X.; Cheng, J.R.; Xiang, Y.B.; Shu, X.O. A case–control study in Shanghai of fruit and vegetable intake and endometrial cancer. Br. J. Cancer 2005, 92, 2059–2064. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.H.; Zheng, W.; Xiang, Y.B.; Ruan, Z.X.; Cheng, J.R.; Dai, Q.; Gao, Y.T.; Shu, X.O. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: Population based case-control study. BMJ 2004, 328, 1285. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.E.; Register, T.C.; Franke, A.A.; Anthony, M.S.; Cline, J.M. Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 2006, 66, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Touillaud, M.; Gelot, A.; Mesrine, S.; Bennetau-Pelissero, C.; Clavel-Chapelon, F.; Arveux, P.; Bonnet, F.; Gunter, M.; Boutron-Ruault, M.-C.; Fournier, A. Use of dietary supplements containing soy isoflavones and breast cancer risk among women aged >50 y: A prospective study. Am. J. Clin. Nutr. 2019, 109, 597–605. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Gao, J.; Wu, X.; Feng, R.; Sun, C. Higher dietary soy intake appears inversely related to breast cancer risk independent of estrogen receptor breast cancer phenotypes. Heliyon 2020, 6, e04228. [Google Scholar] [CrossRef]
- Lee, A.H.; Su, D.; Pasalich, M.; Tang, L.; Binns, C.W.; Qiu, L. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr. Res. 2014, 34, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Messina, M. Insights gained from 20 Years of soy research. J. Nutr. 2010, 140, 2289S–2295S. [Google Scholar] [CrossRef] [Green Version]
- Maskarinec, G.; Ju, D.; Morimoto, Y.; Franke, A.A.; Stanczyk, F.Z. Soy food intake and biomarkers of breast cancer risk: Possible difference in Asian women? Nutr. Cancer 2016, 69, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-Q.; Chen, J.-L.; Liu, Q.; Zhang, Y.; Zeng, H.; Zhao, Y. Soy intake is a with lower endometrial cancer risk: A systematic review and meta-analysis of observational studies. Medicine (Baltimore) 2015, 94, 2281. [Google Scholar] [CrossRef]
- Zhao, T.-T.; Jin, F.; Li, J.-G.; Xu, Y.-Y.; Dong, H.-T.; Liu, Q.; Xing, P.; Zhu, G.-L.; Xu, H.; Miao, Z.-F. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: A meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 136–145. [Google Scholar] [CrossRef]
- Fraser, G.E.; Jaceldo-Siegl, K.; Orlich, M.; Mashchak, A.; Sirirat, R.; Knutsen, S. Dairy, soy, and risk of breast cancer: Those confounded milks. Int. J. Epidemiol. 2020, 49, 1526–1537. [Google Scholar] [CrossRef]
- Zhong, X.-S.; Ge, J.; Chen, S.-W.; Xiong, Y.-Q.; Ma, S.-J.; Chen, Q. Association between dietary isoflavones in soy and legumes and endometrial cancer: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2018, 118, 637–651. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sobue, T.; Kobayashi, M.; Sasaki, S.; Tsugane, S.; Japan Public Health Center-based Prospective Study on Cancer Cardiovascular Diseases Group. Soy, isoflavones, and breast cancer risk in Japan. J. Natl. Cancer Inst. 2003, 95, 906–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362–1375. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Jiang, C. Soy and isoflavones consumption and breast cancer survival and recurrence: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 3079–3090. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose–response meta-analysis. Eur. J. Epidemiol. 2019, 35, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, A.; Barati-Boldaji, R.; Soltani, S.; Mohammadipoor, N.; Esmaeilinezhad, Z.; Clark, C.C.T.; Babajafari, S.; Akbarzadeh, M. Intake of various food groups and risk of breast cancer: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2020, 10, nmaa147. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Coffee drinking and risk of endometrial cancer-A population-based cohort study. Int. J. Cancer 2009, 125, 2413–2417. [Google Scholar] [CrossRef]
- Giri, A.; Sturgeon, S.R.; Luisi, N.; Bertone-Johnson, E.; Balasubramanian, R.; Reeves, K.W. Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: A prospective cohort study among US postmenopausal women. Nutrients 2011, 3, 937–950. [Google Scholar] [CrossRef]
- Gunter, M.J.; Schaub, J.A.; Xue, X.; Freedman, N.D.; Gaudet, M.M.; Rohan, T.E.; Hollenbeck, A.R.; Sinha, R. A prospective investigation of coffee drinking and endometrial cancer incidence. Int. J. Cancer 2012, 131, E530–E536. [Google Scholar] [CrossRef] [Green Version]
- Kotsopoulos, J.; Eliassen, A.E.; Missmer, S.A.; Hankinson, S.E.; Tworoger, S.S. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 2009, 115, 2765–2774. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care 2008, 31, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Willett, W.C.; Hankinson, S.E.; Giovannucci, E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care 2005, 28, 1390–1396. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Quesada, C.; Romanos-Nanclares, A.; Navarro, A.M.; Gea, A.; Cervantes, S.; Martínez-González, M.; Toledo, E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020, 59, 3461–3471. [Google Scholar] [CrossRef]
- Je, Y.; Hankinson, S.E.; Tworoger, S.S.; De Vivo, I.; Giovannucci, E. A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2487–2495. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, L.M.; Johansson, I.; Lenner, P.; Lindahl, B.; Van Guelpen, B. Consumption of filtered and boiled coffee and the risk of incident cancer: A prospective cohort study. Cancer Causes Control. 2010, 21, 1533–1544. [Google Scholar] [CrossRef]
- Arthur, R.; Kirsh, V.A.; Rohan, T.E. Associations of coffee, tea and caffeine intake with risk of breast, endometrial and ovarian cancer among Canadian women. Cancer Epidemiol. 2018, 56, 75–82. [Google Scholar] [CrossRef]
- Lee, P.M.Y.; Chan, W.C.; Kwok, C.C.-H.; Wu, C.; Law, S.-H.; Tsang, K.-H.; Yu, W.-C.; Yeung, Y.-C.; Chang, L.D.J.; Wong, C.K.M.; et al. Associations between coffee products and breast cancer risk: A case-control study in Hong Kong Chinese women. Sci. Rep. 2019, 9, 12684. [Google Scholar] [CrossRef] [Green Version]
- Alicandro, G.; Tavani, A.; La Vecchia, C. Coffee and cancer risk: A summary overview. Eur. J. Cancer Prev. 2017, 26, 424–432. [Google Scholar] [CrossRef]
- Hashibe, M.; Galeone, C.; Buys, S.S.; Gren, L.H.; Boffetta, P.; Zhang, Z.-F.; La Vecchia, C. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br. J. Cancer 2015, 113, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-Z.; Liu, Z.-Q. Chemical kinetic behavior of chlorogenic acid in protecting erythrocyte and DNA against radical-induced oxidation. J. Agric. Food Chem. 2008, 56, 11025–11029. [Google Scholar] [CrossRef]
- Tunnicliffe, J.M.; Shearer, J. Coffee, glucose homeostasis, and insulin resistance: Physiological mechanisms and mediators. Appl. Physiol. Nutr. Metab. 2008, 33, 1290–1300. [Google Scholar] [CrossRef]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef]
- Yuan, J.-M. Cancer prevention by green tea: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 2013, 98, 1676S–1681S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakuta, Y.; Nakaya, N.; Nagase, S.; Fujita, M.; Koizumi, T.; Okamura, C.; Niikura, H.; Ohmori, K.; Kuriyama, S.; Tase, T.; et al. Case-control study of green tea consumption and the risk of endometrial endometrioid adenocarcinoma. Cancer Causes Control. 2009, 20, 617–624. [Google Scholar] [CrossRef]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.-P.; Li, H.; Qiu, Y.-L.; Zhou, G.-M.; Ma, J. Tea consumption and risk of endometrial cancer: A metaanalysis. Am. J. Obstet. Gynecol. 2009, 201, 605.e1–605.e8. [Google Scholar] [CrossRef] [PubMed]
- Man, G.C.W.; Wang, J.; Song, Y.; Wong, J.H.; Zhao, Y.; Lau, T.S.; Leung, K.T.; Chan, T.H.; Wang, H.; Kwong, J.; et al. Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer 2020, 20, 964. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, L.; Zhou, B.; Wang, Y.; Jing, Y.; Wang, B. Alcohol consumption and the risk of endometrial cancer: A meta-analysis. Asia Pac. J. Clin. Nutr. 2011, 20, 125–133. [Google Scholar] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Alcohol intake and endometrial cancer risk: A meta-analysis of prospective studies. Br. J. Cancer 2010, 103, 127–131. [Google Scholar] [CrossRef]
- Neill, A.S.; Nagle, C.M.; Protani, M.M.; Obermair, A.; Spurdle, A.B.; Webb, P.M. For the Australian National Endometrial Cancer Study Group. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: A case-control study, systematic review and meta-analysis. Int. J. Cancer 2013, 132, 1146–1155. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Shi, Z. Association between breastfeeding and endometrial cancer risk: Evidence from a systematic review and meta-analysis. Nutrients 2015, 7, 5697–5711. [Google Scholar] [CrossRef] [Green Version]
- Kitson, S.; Ryan, N.; Mackintosh, M.L.; Edmondson, R.; Duffy, J.M.; Crosbie, E.J. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database Syst. Rev. 2018, 2, CD012513. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J.; Woo, H.D.; Kang, M. Dietary cadmium intake and the risk of cancer: A meta-analysis. PLoS ONE 2013, 8, e75087. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Yang, L.; Sun, Q.; Cong, R.; Gu, H.; Tang, N.; Zhu, H.; Wang, B. Cigarette smoking and the risk of endometrial cancer: A meta-analysis. Am. J. Med. 2008, 121, 501–508.e3. [Google Scholar] [CrossRef] [PubMed]
- Lafranconi, A.; Micek, A.; Galvano, F.; Rossetti, S.; Del Pup, L.; Berretta, M.; Facchini, G. Coffee decreases the risk of endometrial cancer: A dose–response meta-analysis of prospective cohort studies. Nutrients 2017, 9, 1223. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, H.; Zhou, J.-G.; Ma, Y.; Wu, T.; Ma, H. Green tea, black tea consumption and risk of endometrial cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 143–155. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Pasupuleti, V.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Perez-Lopez, F.R. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur. J. Cancer 2015, 51, 2747–2758. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef]
- Zhao, J.; Lyu, C.; Gao, J.; Du, L.; Shan, B.; Zhang, H.; Wang, H.-Y.; Gao, Y. Dietary fat intake and endometrial cancer risk: A dose response meta-analysis. Medicine 2016, 95, e4121. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Giugliano, D. Metabolic syndrome and endometrial cancer: A meta-analysis. Endocrinology 2013, 45, 28–36. [Google Scholar] [CrossRef]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yang, S.; Zhang, J.; Qian, L.; Chen, X. Overweight, obesity and endometrial cancer risk: Results from a systematic review and meta-analysis. Int. J. Biol. Markers 2014, 29, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Behrens, G.; Keimling, M.; Jochem, C.; Ricci, C.; Leitzmann, M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur. J. Epidemiol. 2015, 30, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-J.; Gong, T.-T.; Wang, Y.-Z. Dietary fatty acids intake and endometrial cancer risk: A dose-response meta-analysis of epidemiological studies. Oncotarget 2015, 6, 36081–36097. [Google Scholar] [CrossRef] [Green Version]
- Gifkins, D.; Olson, S.H.; Demissie, K.; Lu, S.-E.; Kong, A.-N.T.; Bandera, E.V. Total and individual antioxidant intake and endometrial cancer risk: Results from a population-based case–control study in New Jersey. Cancer Causes Control 2012, 23, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Kho, P.F.; Glubb, D.M.; Thompson, D.J.; Spurdle, A.B.; O’Mara, T.A. Assessing the role of selenium in endometrial cancer risk: A Mendelian randomization study. Front. Oncol. 2019, 9, 182. [Google Scholar] [CrossRef]
- Ahn, W.-S.; Kim, D.-J.; Chae, G.-T.; Lee, J.-M.; Bae, S.-M.; Sin, J.-I.; Kim, Y.-W.; Namkoong, S.-E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I clinical study of the dietary supplement, Agaricus blazei Murill, in cancer patients in remission. Evid. Based Complement. Altern. Med. 2011, 2011, 192381. [Google Scholar] [CrossRef] [Green Version]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel medicinal mushroom blend as a promising supplement in integrative oncology: A multi-tiered study using 4T1 triple-negative mouse breast cancer model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Hahne, J.C.; Meyer, S.R.; Dietl, J.; Honig, A. The effect of Cordyceps extract and a mixture of Ganoderma lucidum/Agaricus Blazi Murill extract on human endometrial cancer cell lines in vitro. Int. J. Oncol. 2014, 45, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Sexton, E.; Van Themsche, C.; Leblanc, K.; Parent, S.; Lemoine, P.; Asselin, E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol. Cancer 2006, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Bhat, K.P.; Pezzuto, J.M. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res. 2001, 61, 6137–6144. [Google Scholar] [PubMed]
- Dann, J.M.; Sykes, P.H.; Mason, D.R.; Evans, J.J. Regulation of vascular endothelial growth factor in endometrial tumour cells by resveratrol and EGCG. Gynecol. Oncol. 2009, 113, 374–378. [Google Scholar] [CrossRef]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors α and β in endothelial cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef] [Green Version]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Li, Y.; Baer, D.; Friedman, G.D.; Udaltsova, N.; Shim, V.; Klatsky, A.L. Wine, liquor, beer and risk of breast cancer in a large population. Eur. J. Cancer 2009, 45, 843–850. [Google Scholar] [CrossRef]
- Fukuda, T.; Oda, K.; Wada-Hiraike, O.; Sone, K.; Inaba, K.; Ikeda, Y.; Makii, C.; Miyasaka, A.; Kashiyama, T.; Tanikawa, M.; et al. Autophagy inhibition augments resveratrol-induced apoptosis in Ishikawa endometrial cancer cells. Oncol. Lett. 2016, 12, 2560–2566. [Google Scholar] [CrossRef] [Green Version]
- Pavan, A.R.; Da Silva, G.D.B.; Jornada, D.H.; Chiba, D.E.; Fernandes, G.F.D.S.; Chin, C.M.; Dos Santos, J.L. Unraveling the anticancer effect of curcumin and resveratrol. Nutrients 2016, 8, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, A.; Khan, S.; Lee, Y.S. Curcumin molecular targets in obesity and obesity-related cancers. Futur. Oncol. 2012, 8, 179–190. [Google Scholar] [CrossRef]
- Saydmohammed, M.; Joseph, D.; Syed, V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J. Cell. Biochem. 2010, 110, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shah, D.M. Curcumin down-regulates Ets-1 and Bcl-2 expression in human endometrial carcinoma HEC-1-A cells. Gynecol. Oncol. 2007, 106, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, J.; Cui, R.; Lin, J.; Ding, X. Curcumin in treating breast cancer: A Review. J. Lab. Autom. 2016, 21, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Jiao, D.; Dou, M.; Zhang, W.; Lv, L.; Chen, J.; Li, L.; Wang, L.; Han, X. Curcumin inhibits the growth of triple-negative breast cancer cells by silencing EZH2 and restoring DLC1 expression. J. Cell. Mol. Med. 2020, 24, 10648–10662. [Google Scholar] [CrossRef]
- Jahanbakhshi, F.; Dana, P.M.; Badehnoosh, B.; Yousefi, B.; Mansournia, M.A.; Jahanshahi, M.; Asemi, Z.; Halajzadeh, J. Curcumin anti-tumor effects on endometrial cancer with focus on its molecular targets. Cancer Cell Int. 2021, 21, 120. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [Green Version]
- Bradlow, H.L. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. Vivo 2008, 22, 441–445. [Google Scholar] [PubMed]
- Bradlow, H.L.; Telang, N.T.; Sepkovic, D.W.; Osborne, M.P. 2-hydroxyestrone: The ‘good’ estrogen. J. Endocrinol. 1996, 150, S259–S265. [Google Scholar] [PubMed]
- Gupta, M.; McDougal, A.; Safe, S. Estrogenic and antiestrogenic activities of 16α- and 2-hydroxy metabolites of 17β-estradiol in MCF-7 and T47D human breast cancer cells. J. Steroid Biochem. Mol. Biol. 1998, 67, 413–419. [Google Scholar] [CrossRef]
- Liehr, J.G. Is estradiol a genotoxic mutagenic carcinogen? Endocr. Rev. 2000, 21, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Michnovicz, J.J.; Adlercreutz, H.; Bradlow, H.L. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J. Natl. Cancer Inst. 1997, 89, 718–723. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, L.; Chandrasekaran, A.; Liu, K.; Lombardi, S.; Wang, X.-P.; Auborn, K.J.; Goodwin, L. Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol. Med. 2007, 13, 69–78. [Google Scholar] [CrossRef]
- Leong, H.; Firestone, G.L.; Bjeldanes, L.F. Cytostatic effects of 3,3’-diindolylmethane in human endometrial cancer cells result from an estrogen receptor-mediated increase in transforming growth factor-α expression. Carcinogenesis 2001, 22, 1809–1817. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, B.; Shin, S.-J.; Kwon, S.-H.; Cha, S.-D.; Lee, H.-G.; Bae, I.; Cho, C.-H. The synergistic apoptotic interaction of indole-3-carbinol and genistein with TRAIL on endometrial cancer cells. J. Korean Med Sci. 2013, 28, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Kojima, T.; Tanaka, T.; Mori, H. Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res. 1994, 54, 1446–1449. [Google Scholar] [PubMed]
- Barrenetxe, J.; Delagrange, P.; Martínez, J.A. Physiological and metabolic functions of melatonin. J. Physiol. Biochem. 2004, 60, 61–72. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Cos, S.; Mediavilla, D.; Martínez-Campa, C.; González, A.; Alonso-González, C. Melatonin-estrogen interactions in breast cancer. J. Pineal Res. 2005, 38, 217–222. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. Role and therapeutic potential of melatonin in various type of cancers. Onco. Targets Ther. 2021, 14, 2019–2052. [Google Scholar] [CrossRef]
- Vitti-Ruela, B.V.; Dokkedal-Silva, V.; Hachul, H.; Tufik, S.; Andersen, M.L. Melatonin and vitamin D: Complementary therapeutic strategies for breast cancer. Support. Care Cancer 2021. [Google Scholar] [CrossRef]
- Lissoni, P.; Chilelli, M.; Villa, S.; Cerizza, L.; Tancini, G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: A randomized trial. J. Pineal Res. 2003, 35, 12–15. [Google Scholar] [CrossRef]
- Lissoni, P.; Malugani, F.; Brivio, F.; Piazza, A.; Vintimilla, C.; Giani, L.; Tancini, G. Total pineal endocrine substitution therapy (TPEST) as a new neuroendocrine palliative treatment of untreatable metastatic solid tumor patients: A phase II study. Neuro Endocrinol. Lett. 2003, 24, 259–262. [Google Scholar] [PubMed]
- Reiter, R.J. Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 2004, 37, 213–214. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Tan, D.-X.; León, J.; Manchester, L.; Reiter, R.J. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate 2005, 63, 29–43. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Therapeutic actions of melatonin in cancer: Possible mechanisms. Integr. Cancer Ther. 2008, 7, 189–203. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.-X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.-F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Campa, C.; González, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Alvarez-Garcia, V.; Sánchez-Barceló, E.J.; Cos, S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br. J. Cancer 2009, 101, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Tan, D.-X.; Reiter, R.J. Melatonin: A versatile protector against oxidative DNA damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondy, S.C.; Campbell, A. Mechanisms underlying tumor suppressive properties of melatonin. Int. J. Mol. Sci. 2018, 19, 2205. [Google Scholar] [CrossRef] [Green Version]
- Littman, A.J.; Beresford, S.A.; White, E. The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control. 2001, 12, 691–702. [Google Scholar] [CrossRef]
- Gonzalez, C.A. The European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2006, 9, 124–126. [Google Scholar] [CrossRef] [Green Version]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Cust, A.E.; Slimani, N.; Kaaks, R.; Van Bakel, M.; Biessy, C.; Ferrari, P.; Laville, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Dietary carbohydrates, glycemic index, glycemic load, and endometrial cancer risk within the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Epidemiol. 2007, 166, 912–923. [Google Scholar] [CrossRef]
- Aarestrup, J.; Kyrø, C.; Christensen, J.; Kristensen, M.; Würtz, A.M.L.; Johnsen, N.F.; Overvad, K.; Tjønneland, A.; Olsen, A. Whole grain, dietary fiber, and incidence of endometrial cancer in a Danish cohort study. Nutr. Cancer 2012, 64, 1160–1168. [Google Scholar] [CrossRef]
- Koutoukidis, D.; Beeken, R.J.; Manchanda, R.; Burnell, M.; Ziauddeen, N.; Michalopoulou, M.; Knobf, M.T.; Lanceley, A. Diet, physical activity, and health-related outcomes of endometrial cancer survivors in a behavioral lifestyle program: The Diet and Exercise in Uterine Cancer Survivors (DEUS) parallel randomized controlled pilot trial. Int. J. Gynecol. Cancer 2019, 29, 531–540. [Google Scholar] [CrossRef]
- Haggerty, A.F.; Hagemann, A.; Barnett, M.; Thornquist, M.; Neuhouser, M.L.; Horowitz, N.; Colditz, G.A.; Sarwer, D.B.; Ko, E.M.; Allison, K.C. A randomized, controlled, multicenter study of technology-based weight loss interventions among endometrial cancer survivors. Obesity 2017, 25, S102–S108. [Google Scholar] [CrossRef] [Green Version]
- Demark-Wahnefried, W.; Cases, M.G.; Cantor, A.B.; Frugé, A.D.; Smith, K.P.; Locher, J.; Cohen, H.J.; Tsuruta, Y.; Daniel, M.; Kala, R.; et al. Pilot randomized controlled trial of a home vegetable gardening intervention among older cancer survivors shows feasibility, satisfaction, and promise in improving vegetable and fruit consumption, reassurance of worth, and the trajectory of central adiposity. J. Acad. Nutr. Diet. 2018, 118, 689–704. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Lopes, S.; Atkins, L.; Croker, H.; Knobf, M.T.; Lanceley, A.; Beeken, R.J. Use of intervention mapping to adapt a health behavior change intervention for endometrial cancer survivors: The shape-up following cancer treatment program. BMC Public Health 2018, 18, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Gruenigen, V.E.; Gibbons, H.E.; Kavanagh, M.B.; Janata, J.W.; Lerner, E.; Courneya, K.S. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: Quality of life outcomes and mediators of behavior change. Health Qual. Life Outcomes 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Gruenigen, V.E.; Waggoner, S.E.; Frasure, H.E.; Kavanagh, M.B.; Janata, J.W.; Rose, P.G.; Courneya, K.S.; Lerner, E. Lifestyle challenges in endometrial cancer survivorship. Obstet. Gynecol. 2011, 117, 93–100. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science—implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Zanini, B.; Simonetto, A.; Bertolotti, P.; Marullo, M.; Marconi, S.; Becchetti, C.; Gilioli, G.; Valerio, A.; Donato, F.; Ricci, C.; et al. A new self-administered semi-quantitative food frequency questionnaire to estimate nutrient intake among Italian adults: Development design and validation process. Nutr. Res. 2020, 80, 18–27. [Google Scholar] [CrossRef]
- Patterson, R.E.; Kristal, A.R.; Tinker, L.F.; Carter, R.A.; Bolton, M.P.; Agurs-Collins, T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999, 9, 178–187. [Google Scholar] [CrossRef]
- Hislop, T.G.; Lamb, C.W.; Ng, V.T. Differential misclassification bias and dietary recall for the distant past using a food frequency questionnaire. Nutr. Cancer 1990, 13, 223–233. [Google Scholar] [CrossRef]
- Pierce, B.L.; Kraft, P.; Zhang, C. Mendelian randomization studies of cancer risk: A literature review. Curr. Epidemiol. Rep. 2018, 5, 184–196. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The challenge of reforming nutritional epidemiologic research. JAMA 2018, 320, 969–970. [Google Scholar] [CrossRef]
- Yu, C.-H.; Wang, T.-J.; Chang, C.-L.; Liang, S.-Y.; Wu, S.-F.; Liu, C.-Y.; Lu, Y.Y. Healthy life styles, sleep and fatigue in endometrial cancer survivors: A cross-sectional study. J. Clin. Nurs. 2020, 29, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Coburn, S.B.; Moore, S.C.; Brinton, L.A.; Wentzensen, N.; Anderson, G.; Wallace, R.; Falk, R.T.; Pfeiffer, R.; Xu, X.; et al. Alcohol and oestrogen metabolites in postmenopausal women in the Women’s Health Initiative Observational Study. Br. J. Cancer 2018, 118, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Population | Incidence | Prevalence | Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Number | ** Crude Rate | ** Age-Standardised Rate | 5-Year | ** Proportions | Number | ** Crude Rate | ** Age-Standardised Rate | |
| High Income | 173,957 | 28.5 | 15.5 | 670,689 | 109.8 | 36,911 | 6.0 | 2.4 |
| Upper Middle Income | 146,537 | 11.3 | 8.2 | 451,728 | 34.7 | 31,725 | 2.4 | 1.6 |
| Lower Middle Income | 52,535 | 3.6 | 3.9 | 140,955 | 9.5 | 17,632 | 1.2 | 1.3 |
| Low Income | 7,169 | 1.9 | 3.2 | 13,968 | 3.7 | 3,331 | 0.88 | 1.6 |
| Factor | Type 1 EC | Type 2 EC | References |
|---|---|---|---|
| Frequency distribution | 80–90% | 10–20% | [71,74] |
| Prognosis | Favourable | Unfavourable | |
| Outcome (5-year survival) | 86% | 59% | |
| Onset of menopause | ≥50 years of age | ≤50 years of age | |
| Obesity, hyperlipidaemia and diabetes mellitus association | Yes | No | |
| Association with oestrogen stimulation | Yes | No | |
| Sensitivity to progestagens | High | Low | |
| Background endometrium histology | Hyperplasia | Atrophy | |
| Tumour grade | Low (grade 1–2) | High (grade 3) | |
| Myometrial invasion | Superficial | Deep | |
| Potential for metastatic spread through lymphatic system | Low | High | |
| Reproductive status | Decreased | No disturbance | |
| Clinicopathologic and molecular alterations | |||
| Prototypical histological type | Endometrioid | Non-endometrioid (serous, clear cell carcinoma) | [71,75,76,77,78] |
| Stage at diagnosis | Early (FIGO stage I–II) | Advanced (FIGO stage III–IV) | |
| ER and PR receptor expression | High | Low | |
| Common genetic alterations | |||
| Microsatellite instability * | 28–40% | 0–2% | [66,70,71,79,80,81,82,83] |
| PTEN mutation | 52–78% | 1–11% | |
| TP53 mutation | 9–12% | 60–91% | |
| POLE mutation | 11–20% | 0–7% | |
| KRAS mutation | 15–43% | 2–8% | |
| PIK3CA mutation | 36–52% | 24–42% | |
| PIK3R1 mutation | 21–43% | 0–12% | |
| ARID1A mutation | 25–48% | 6–11% | |
| CTNNB1 mutation | 23–24% | 0–3% | |
| PPP2R1A mutation | 5–7% | 15–43% | |
| ERRB2 mutation | 1–4% | 26–44% | |
| HER2 amplification | 0 | 27–44% | |
| Factor | Ref | Studies Included | Odds Ratio or Relative Risk (95% CI) | Direction of Risk |
|---|---|---|---|---|
| Alcohol a | [319] [320] | 14 case-control 7 cohort design |
0.89 (0.76–1.05) 1.17 (0.93–1.46) | No effect No effect |
| Aspirin b | [321] | 5 case-control 4 cohort design Overall |
0.82 (0.71–0.96) 0.91 (0.80–1.03) 0.87 (0.79–0.96) | ↓ No effect ↓ |
| Beast feeding | [322] | 4 prospective and 10 retrospective studies | 0.77 (0.62–0.96) | ↓ |
| Body mass index c | [323] | Randomised clinical trial | RR 19.03 (1.17–310.52) | ↑ |
| Cadmium d | [324] | 4 cohort design | 1.40 (1.06–1.84) | ↑ |
| Calcium | [255] | 3 case-control | 0.62 (0.42–0.93) | ↓ |
| Cholesterol | [109] | 9 case-control 1 cohort design |
1.07 (1.01–1.13) 1.06 (1.00–1.12) | ↑ ↑ |
| Cigarette Smoking | [325] | 24 case-control 10 prospective | 0.72 (0.66–0.79) 0.81 (0.74–0.88) | ↓ ↓ |
| Coffee (caffeinated vs. de-caffeinated) | [326] | 10 case-control 4 cohort design |
0.79 (0.73, 0.87) 0.65 (0.50, 0.85) e 0.76 (0.62, 0.93) f | ↓ ↓ ↓ |
| Dairy products | [196] | 8 case-control | 0.97 (0.93–1.01) | No effect |
| Green tea | [316] [327] | 7 case-control 6 cohort design | 0.78 (0.66–0.92) 0.97 (0.82–1.14) | ↓ No effect |
| Insulin resistance g | [328] | 2 case-control 2 prospective 21 cross-sectional |
37.52 pmol/L (16.64–58.40) 33.94 pmol/L (15.04–52.85) 21.25 pmol/L (1.45–41.05) | ↑ ↑ ↑ |
| Lignans | [329] | 3 case-control | 0.92 (0.71, 1.20) | No effect |
| Lipids | [330] | 14 case-control 7 cohort | 1.21 (1.01–1.45) 0.91 (0.83–1.01) | ↑ No effect |
| Metabolic syndrome | [331] | 3 case-control 1 nested case-control 1 prospective 1 cohort registry Overall |
2.45 (1.81–3.08) 1.62 (1.08–2.42) 1.37 (1.28–1.46) 2.77 (1.74–4.40) 1.89 (1.34–2.67) | ↑ ↑ ↑ ↑ ↑ |
| Oestrogen only HRT | [27] [332] | 1 case-control 30 cohort design |
1.45 [1.02–2.06] 2.30 (2.10–2.50) | ↑ ↑ |
| Oestrogen plus progestagens HRT | [332] | 30 cohort design | 0.40 (0.20–0.60) | ↓ |
| Overweight/Obesity h | [333] | 8 case-control 6 cohort design 8 case-control h 6 cohort design h |
1.18 (0.96–1.45) 1.47 (1.22–1.78) 2.06 (1.61–2.63) 3.11 (2.62–3.69) | No effect ↑ ↑ ↑ |
| Physical activity | [334] | 14 case-control 19 cohort design |
RR 0.72 (0.64–0.80) RR 0.84 (0.78–0.91) | ↓ ↓ |
| Red Meat | [196] | 7 case-control | 1.60 (1.26–2.03) | ↑ |
| Saturated fatty acids | [335] | 10 case-control 4 cohort design |
RR 1.06 (0.98–1.14) RR 0.97 (0.93–1.00) | No effect No effect |
| Selenium | [336] [337] | 1 case-control 1 cohort design | 0.74 (0.47–1.17) 0.90 (0.53–1.50) | No effect ↓ |
| Soy isoflavones | [329] | 5 case-control 2 prospective | 0.82 (0.69, 0.97) 0.80 (0.51, 1.26) | ↓ No effect |
| Vitamin A i | [220] | 12 case-control 1 cohort | 0.88 (0.79–0.98) 0.94 (0.84–1.05) | ↓ No effect |
| Vitamin C | [220] | 12 case-control 1 cohort | 0.85 (0.73–0.98) 1.01 (0.79–1.28) | ↓ No effect |
| Vitamin D | [220] | 3 case-control | 0.85 (0.34–2.13) | No effect |
| Vitamin E | [220] | 12 case-control 1 cohort | 0.91 (0.84–0.99) 1.06 (0.82–1.37) | ↓ No effect |
| Somatic Change | Gene Product and Function | Classification Frequencies * |
|---|---|---|
| Mutated PTEN |
Phosphatase And Tensin Homolog Tumour suppressor gene that antagonises the PI3K-AKT/PKB signalling pathway |  |
| Mutated ARID1A |
AT-Rich Interactive Domain-Containing Protein 1A A component of SWI/SNF chromatin remodelling complexes that represses expression of select genes through changing chromatin structure |  |
| Mutated PIK3CA |
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha Participates in cellular signalling in response to various growth factors and is involved in the activation of AKT1-PDPK1 signalling cascades involved in cell growth and survival. |  |
| Mutated PIK3R1 | Phosphoinositide-3-Kinase Regulatory Subunit 1 Acts as an adapter, mediating the association of the p110 catalytic unit to the plasma membrane, which is necessary for the insulin-stimulated increase in glucose uptake and glycogen synthesis in insulin-sensitive tissues. |  |
| Mutated KRAS | KRAS Proto-Oncogene, GTPase Plays an important role in the regulation of cell proliferation by promoting oncogenic events by inducing transcriptional silencing of tumour suppressor genes. |  |
| Mutated CTNNB1 |
Catenin Beta 1 A key component in the Wnt signalling pathway and in insulin internalisation. When mutated, it accumulates in the nucleus, where it acts as a coactivator for transcription factors of the TCF/LEF family, leading to activation of Wnt responsive genes. |  |
| Microsatellite Instability (MSI) | This genetic aberration is associated with dysfunctional DNA mismatch repair mechanisms. The major protein product MLH1 interacts with PMS2 to introduce single-strand breaks near the DNA mismatch and thus generates new entry points for the exonuclease EXO1 to degrade the strand containing the mismatch. |  |
| Mutated FGFR2 | Fibroblast Growth Factor Receptor 2 Plays an essential role in the regulation of cell proliferation, differentiation, migration and apoptosis and mediates activation of RAS, MAPK1/ERK2, MAPK3/ERK1 and the MAP kinase signalling pathway, as well as the AKT1 signalling pathway. |  |
| Mutated POLE | DNA Polymerase Epsilon, Catalytic Subunit Catalytic component of the DNA polymerase epsilon complex and participates in chromosomal DNA replication. It has 3’-5’ proofreading exonuclease activity that corrects errors arising during DNA replication and so aids DNA synthesis during DNA repair. |  |
| Mutated TP53 | Tumor Protein P53 Acts as a tumour suppressor in many tumour types; induces growth arrest or apoptosis depending on the physiological circumstances and cell type. Apoptosis induction is mediated either by stimulation of BAX and FAS antigen expression, or by repression of Bcl-2 expression. |  |
| Mutated FBWX7 | F-Box And WD Repeat Domain Containing 7 Substrate recognition component of a SCF (SKP1-CUL1-F-box protein) E3 ubiquitin-protein ligase complex which mediates the ubiquitination and subsequent proteasomal degradation of target proteins involved in cell cycle control. |  |
| Mutated PPP2R1A | Protein Phosphatase 2 Scaffold Subunit A alpha Required for proper chromosome segregation and for centromeric localisation of shugoshin 1 (SGO1) in mitosis, resulting in increased cell proliferation. |  |
| Amplified ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 In the nucleus, this protein inhibits the transcription of Cyclin Dependent Kinase Inhibitor 1A (CDKN1A), a key regulator of the cell cycle and TP53 mediated inhibition of cellular proliferation in response to DNA damage. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. https://doi.org/10.3390/cancers13092149
Yasin HK, Taylor AH, Ayakannu T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers. 2021; 13(9):2149. https://doi.org/10.3390/cancers13092149
Chicago/Turabian StyleYasin, Hajar Ku, Anthony H. Taylor, and Thangesweran Ayakannu. 2021. "A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer" Cancers 13, no. 9: 2149. https://doi.org/10.3390/cancers13092149