Molecular Insights into the Thrombotic and Microvascular Injury in Placental Endothelium of Women with Mild or Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Selection and Specimens
2.3. RT-PCR for Placental SARS-CoV-2 Infection
2.4. Immunofluorescence
2.5. Histochemical Staining and Hofbauer Cell Assessment
2.6. Statistical Analysis
3. Results
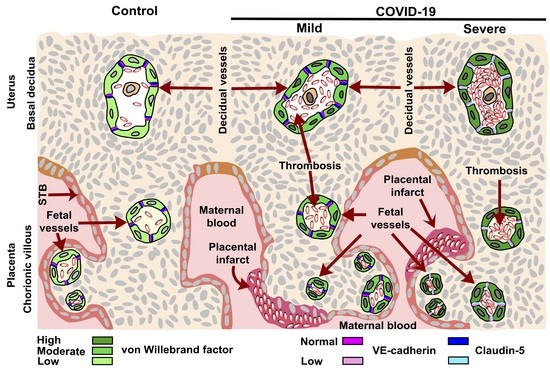
3.1. vWf Is Overexpressed in the Endothelium of Decidua and Chorionic Villi of Placentas Derived from Women with COVID-19 according to Disease Severity
3.2. The Expression of Claudin-5 Diminishes in the Endothelium of Decidua and Chorionic Villi of Placentas from Women with Severe COVID-19
3.3. VE-Cadherin Expression Diminishes in Decidua and Chorionic Villi Endothelium of Placentas from Women with Severe COVID-19
3.4. Placentas of Women with COVID-19 Display Histological Alterations Indicative of Vasculopathy and a Higher Number of Hofbauer Cells Is Observed in Placentas from Women with Severe COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaraj, S.H.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J. Microbiol. Immunol. Infect. 2019, 52, 501–503. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA 2020, 323. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020, 4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breslin, N.; Baptiste, C.; Miller, R.; Fuchs, K.; Goffman, D.; Gyamfi-Bannerman, C.; D’Alton, M. Coronavirus disease 2019 in pregnancy: Early lessons. Am. J. Obstet. Gynecol. MFM 2020, 2, 100111. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1635–1640. [Google Scholar] [CrossRef]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; La Rosa, M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, 37, 861–865. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guo, L.; Chen, L.; Liu, W.; Cao, Y.; Zhang, J.; Feng, L. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis. 2020, 71. [Google Scholar] [CrossRef]
- Zeng, L.; Xia, S.; Yuan, W.; Yan, K.; Xiao, F.; Shao, J.; Zhou, W. Neonatal Early-Onset Infection With SARS-CoV-2 in 33 Neonates Born to Mothers With COVID-19 in Wuhan, China. JAMA Pediatr. 2020, 174. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Duran, P.; Berman, S.; Niermeyer, S.; Jaenisch, T.; Forster, T.; Gomez Ponce de Leon, R.; De Mucio, B.; Serruya, S. COVID-19 and newborn health: Systematic review. Rev. Panam. Salud Publica 2020, 44, e54. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, J.; Mo, Y.; Duan, W.; Xiang, G.; Yi, M.; Bao, L.; Shi, Y. Unlikely SARS-CoV-2 vertical transmission from mother to child: A case report. J. Infect. Public Health 2020, 13, 818–820. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, 192, E647–E650. [Google Scholar] [CrossRef]
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.; Roman, A.S. Detection of SARS-COV-2 in Placental and Fetal Membrane Samples. Am. J. Obstet. Gynecol. MFM 2020, 100133. [Google Scholar] [CrossRef] [PubMed]
- Ferraiolo, A.; Barra, F.; Kratochwila, C.; Paudice, M.; Vellone, V.G.; Godano, E.; Varesano, S.; Noberasco, G.; Ferrero, S.; Arioni, C. Report of Positive Placental Swabs for SARS-CoV-2 in an Asymptomatic Pregnant Woman with COVID-19. Medicina (Kaunas) 2020, 56, 306. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Patane, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 100145. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223. [Google Scholar] [CrossRef]
- Kniss, D.A. Letter-to-the-Editor: Alternative Interpretation to the Findings Reported in Visualization of SARS-CoV-2 Virus Invading the Human Placenta Using Electron Microscopy. Am. J. Obstet. Gynecol. 2020, 223. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2020, 184. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Pohlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pringle, K.G.; Tadros, M.A.; Callister, R.J.; Lumbers, E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkruys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Colmenero, I.; Santonja, C.; Alonso-Riano, M.; Noguera-Morel, L.; Hernandez-Martin, A.; Andina, D.; Wiesner, T.; Rodriguez-Peralto, J.L.; Requena, L.; Torrelo, A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultraestructural study of 7 paediatric cases. Br. J. Dermatol. 2020, 183. [Google Scholar] [CrossRef]
- Santonja, C.; Heras, F.; Nunez, L.; Requena, L. COVID-19 chilblain-like lesion: Immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a PCR-negative patient. Br. J. Dermatol. 2020, 183. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Hernandez-Fernandez, F.; Valencia, H.S.; Barbella-Aponte, R.A.; Collado-Jimenez, R.; Ayo-Martin, O.; Barrena, C.; Molina-Nuevo, J.D.; Garcia-Garcia, J.; Lozano-Setien, E.; Alcahut-Rodriguez, C.; et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- South, K.; Lane, D.A. ADAMTS-13 and von Willebrand factor: A dynamic duo. J. Thromb. Haemost. 2018, 16, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Schwameis, M.; Schorgenhofer, C.; Assinger, A.; Steiner, M.M.; Jilma, B. VWF excess and ADAMTS13 deficiency: A unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb. Haemost. 2015, 113, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Morici, N.; Bottiroli, M.; Fumagalli, R.; Marini, C.; Cattaneo, M. Role of von Willebrand Factor and ADAMTS-13 in the Pathogenesis of Thrombi in SARS-CoV-2 Infection: Time to Rethink. Thromb. Haemost. 2020, 120. [Google Scholar] [CrossRef] [PubMed]
- Huisman, A.; Beun, R.; Sikma, M.; Westerink, J.; Kusadasi, N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020, 42. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Escher, R.; Breakey, N.; Lammle, B. ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb. Res. 2020, 192, 174–175. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Breakey, N.; Escher, R. D-dimer and mortality in COVID-19: A self-fulfilling prophecy or a pathophysiological clue? Swiss Med. Wkly. 2020, 150, w20293. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.V.; Fleming, P.A.; Argraves, W.S.; Corada, M.; Zanetta, L.; Dejana, E.; Drake, C.J. VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood 2005, 105, 2771–2776. [Google Scholar] [CrossRef] [Green Version]
- Gory-Faure, S.; Prandini, M.H.; Pointu, H.; Roullot, V.; Pignot-Paintrand, I.; Vernet, M.; Huber, P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development 1999, 126, 2093–2102. [Google Scholar] [PubMed]
- Carmeliet, P.; Lampugnani, M.G.; Moons, L.; Breviario, F.; Compernolle, V.; Bono, F.; Balconi, G.; Spagnuolo, R.; Oosthuyse, B.; Dewerchin, M.; et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 1999, 98, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Corada, M.; Liao, F.; Lindgren, M.; Lampugnani, M.G.; Breviario, F.; Frank, R.; Muller, W.A.; Hicklin, D.J.; Bohlen, P.; Dejana, E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 2001, 97, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Abdelhalim, N.Y.; Shehata, M.H.; Gadallah, H.N.; Sayed, W.M.; Othman, A.A. Morphological and ultrastructural changes in the placenta of the diabetic pregnant Egyptian women. Acta Histochem. 2018, 120, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cordero, M.; Bosco, C.; Gonzalez, J.; Gutierrez, R.; Barja, P.; Rodrigo, R. Immunohistochemical expression of von Willebrand factor in the preeclamptic placenta. J. Mol. Histol. 2011, 42, 459–465. [Google Scholar] [CrossRef]
- Shahidi, M. Thrombosis and von Willebrand Factor. Adv. Exp. Med. Biol. 2017, 906, 285–306. [Google Scholar] [CrossRef]
- Goksever Celik, H.; Uhri, M.; Yildirim, G. Expression of von Willebrand factor and caldesmon in the placental tissues of pregnancies complicated with intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2019, 32, 916–921. [Google Scholar] [CrossRef]
- Aref, S.; Goda, H. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematology 2013, 18, 237–241. [Google Scholar] [CrossRef]
- Bosco, C.; Gonzalez, J.; Gutierrez, R.; Parra-Cordero, M.; Barja, P.; Rodrigo, R. Oxidative damage to pre-eclamptic placenta: Immunohistochemical expression of VEGF, nitrotyrosine residues and von Willebrand factor. J. Matern. Fetal Neonatal Med. 2012, 25, 2339–2345. [Google Scholar] [CrossRef]
- Lievano, S.; Alarcon, L.; Chavez-Munguia, B.; Gonzalez-Mariscal, L. Endothelia of term human placentae display diminished expression of tight junction proteins during preeclampsia. Cell Tissue Res. 2006, 324, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Martin-Tapia, D.; Valdespino-Vazquez, Y.; Alarcon, L.; Espejel-Nunez, A.; Guzman-Huerta, M.; Munoz-Medina, J.E.; Shibayama, M.; Chavez-Munguia, B.; Estrada-Gutierrez, G.; et al. Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability. Cells 2019, 8, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challier, J.C.; Dubernard, G.; Galtier, M.; Bintein, T.; Vervelle, C.; Raison, D.; Espie, M.J.; Uzan, S. Junctions and adhesion molecules in first trimester and term human placentas. Cell Mol. Biol. 2005, 51. [Google Scholar] [CrossRef] [Green Version]
- Vailhe, B.; Kapp, M.; Dietl, J.; Arck, P. Human first-trimester decidua vascular density: An immunohistochemical study using VE-cadherin and endoglin as endothelial cell markers. Am. J. Reprod. Immunol. 2000, 44, 9–15. [Google Scholar] [CrossRef]
- Leach, L.; Clark, P.; Lampugnani, M.G.; Arroyo, A.G.; Dejana, E.; Firth, J.A. Immunoelectron characterisation of the inter-endothelial junctions of human term placenta. J. Cell Sci. 1993, 104 Pt 4, 1073–1081. [Google Scholar]
- Li, Y.; Zhao, Y.J.; Zou, Q.Y.; Zhang, K.; Wu, Y.M.; Zhou, C.; Wang, K.; Zheng, J. Preeclampsia does not alter vascular growth and expression of CD31 and vascular endothelial cadherin in human placentas. J. Histochem. Cytochem. 2015, 63, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groten, T.; Gebhard, N.; Kreienberg, R.; Schleussner, E.; Reister, F.; Huppertz, B. Differential expression of VE-cadherin and VEGFR2 in placental syncytiotrophoblast during preeclampsia—New perspectives to explain the pathophysiology. Placenta 2010, 31, 339–343. [Google Scholar] [CrossRef]
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial Dysfunction in Diabetes. Biomedicines 2020, 8, 182. [Google Scholar] [CrossRef]
- Tooke, J.E. Microvascular function in human diabetes. A physiological perspective. Diabetes 1995, 44, 721–726. [Google Scholar] [CrossRef]
- Babawale, M.O.; Lovat, S.; Mayhew, T.M.; Lammiman, M.J.; James, D.K.; Leach, L. Effects of gestational diabetes on junctional adhesion molecules in human term placental vasculature. Diabetologia 2000, 43, 1185–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumuller, S.; Lehnen, H.; Schmitz, J.; Fimmers, R.; Muller, A.M. The impact of insulin treatment on the expression of vascular endothelial cadherin and Beta-catenin in human fetoplacental vessels. Pediatr. Dev. Pathol. 2015, 18, 17–23. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Mulvey, J.J.; Magro, C.M.; Ma, L.X.; Nuovo, G.J.; Baergen, R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020, 46, 151530. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef]
- Suzuki, K.; Itoh, H.; Kimura, S.; Sugihara, K.; Yaguchi, C.; Kobayashi, Y.; Hirai, K.; Takeuchi, K.; Sugimura, M.; Kanayama, N. Chorangiosis and placental oxygenation. Congenit. Anom. (Kyoto) 2009, 49, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Reyes, L.; Golos, T.G. Hofbauer Cells: Their Role in Healthy and Complicated Pregnancy. Front. Immunol. 2018, 9, 2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.M.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like syndrome induced by severe COVID-19: A prospective observational study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1374–1380. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Nakashima, A.; Huber, W.J.; Davis, S.; Banerjee, S.; Huang, Z.; Saito, S.; Sadovsky, Y.; Sharma, S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2020, 31116–31119. [Google Scholar] [CrossRef]
- Mathias, M.; Bitar, M.; Aldulescu, M.; Birkett, R.; Perez, M.; Mestan, K. Placental vascular maldevelopment, intrauterine growth restriction, and pulmonary hypertension. Pulm. Circ. 2020, 10. [Google Scholar] [CrossRef]
- Heider, A. Fetal Vascular Malperfusion. Arch. Pathol. Lab. Med. 2017, 141, 1484–1489. [Google Scholar] [CrossRef] [Green Version]
- Starikov, R.; Has, P.; Wu, R.; Nelson, D.M.; He, M. Small-for-gestational age placentas associate with an increased risk of adverse outcomes in pregnancies complicated by either type I or type II pre-gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2020, 1–6. [Google Scholar] [CrossRef]
- Gulersen, M.; Prasannan, L.; Tam Tam, H.; Metz, C.N.; Rochelson, B.; Meirowitz, N.; Shan, W.; Edelman, M.; Millington, K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM 2020, 2, 100211. [Google Scholar] [CrossRef]








| Cnt1 | Cnt2 | Cnt3 | Cnt4 | |
|---|---|---|---|---|
| Maternal Age (years) | 24 | 17 | 17 | 20 |
| Maternal comorbidity | None | None | None | None |
| Maternal weight (kg) | NA | 53.7 | 62.1 | 65.0 |
| Maternal height (m) | NA | 1.67 | 1.64 | 1.59 |
| GA at Diagnosis (weeks) GA at Delivery | 13.6 | 38.1 | 40.2 | 30.3 |
| (weeks) | 13.6 | 38.1 | 40.2 | 30.3 |
| Delivery mode | Curettage | CS | CS | CS |
| NB weight (g) | NA | 2315 | 3170 | 1320 |
| NB Gender | NA | Male | Female | Female |
| NB weight classification | NA | SGA | AGA | AGA |
| White blood cell count/UL | 7500 | 6600 | 10,500 | 11,300 |
| Lymphocyte count (%) | 9.0 | 15.1 | 10.1 | 9.8 |
| Platelets cells/UL | 187,000 | 236,000 | 274,000 | 167,000 |
| Thrombin time (s) | 11.0 | 9.5 | 10.9 | 10.8 |
| Prothrombin time | 26.9 | 24.9 | 36.2 | 29.6 |
| mCV1 | mCV2 | mCV3 | mCV4 | mCV5 | |
|---|---|---|---|---|---|
| Maternal Age (years) | 28 | 20 | 34 | 19 | 20 |
| Maternal comorbidity | None | None | Epilepsy | None | None |
| Maternal weight (kg) | NA | 65.4 | 93.7 | 75.0 | 57.0 |
| Maternal height (m) | NA | 1.69 | 1.67 | 1.59 | 1.50 |
| COVID-19 stage | Acute | Acute | Acute | Acute | Acute |
| PCR Mother | + | + | + | + | + |
| PCR NB/Fetal | + | + | + | + | + |
| PCR Placenta | − | − | − | − | − |
| COVID-19 symptoms | Cough Headache | None | None | None | None |
| GA at Diagnosis (weeks) GA at Delivery | 13.0 | 40.3 | 39.5 | 33.4 | 26.4 |
| (weeks) | 13.0 | 40.4 | 39.5 | 33.5 | 26.6 |
| Delivery mode | Curettage | CS | CS | CS | CS |
| NB weight (g) | NA | 3410 | 3615 | 1946 | 978 |
| NB Gender | NA | Male | Male | Male | Female |
| NB weight classification | NA | AGA | LGA | AGA | AGA |
| White blood cell count/UL | NA | 8400 | 7900 | 10,200 | 107,700 |
| Lymphocyte count (%) | NA | 30.1 | 17 | 2.9 | 11 |
| Platelets cells/UL | NA | 224,000 | 208,000 | 177,000 | 341,000 |
| Thrombin time (s) | NA | 9.2 | 10.3 | 10.8 | 10.3 |
| Prothrombin time | NA | 29.8 | 23.8 | 28.6 | 30.4 |
| sCV1 | sCV2 | sCV3 | sCV4 | sCV5 | sCV6 | |
|---|---|---|---|---|---|---|
| Maternal Age (years) | 37 | 25 | 25 | 37 | 36 | 39 |
| Maternal comorbidity | None | None | None | None | None | None |
| Maternal weight (kg) | 91 | 67 | 66 | 60 | 75.5 | 77.5 |
| Maternal height (m) | 1.65 | 1.55 | 1.60 | 1.50 | 1.60 | 1.64 |
| COVID-19 stage | Acute | Acute | Acute | Acute | Acute | Acute |
| PCR Mother | + | + | + | + | + | + |
| PCR NB/Fetal | − | − | − | − | − | − |
| PCR Placenta | − | − | − | − | − | − |
| COVID-19 symptoms | Dyspnea, myalgias, arthralgia, diarrhea | Cough, fever, dyspnea, myalgias, arthralgias, rhinorrhea | Cough, fever, dyspnea, myalgias, arthralgias, diarrhea, rhinorrhea | Cough, fever, myalgias, arthralgias | Cough, fever, dyspnea | Cough, fever, dyspnea |
| GA at Diagnosis (weeks) | 27.6 | 34.6 | 28.0 | 38 | 39.1 | 39.1 |
| GA at Delivery (weeks) | 27.6 | 34.6 | 28.0 | 38 | 39.1 | 39.1 |
| Delivery mode | CS | CS | CS | CS | CS | CS |
| NB weight (g) | 1600 | 2200 | 1250 | 2640 | 2330 | 2900 |
| NB Gender | Female | Female | Female | Female | Female | Male |
| NB weight classification | AGA | AGA | AGA | AGA | SGA | AGA |
| White blood cell count/UL | 11,200 | 8900 | 16,500 | 7600 | 9900 | 8800 |
| Lymphocyte count (%) | 16.3 | 9.5 | 4.7 | 24.2 | 24.1 | 24.0 |
| Platelets cells/UL | 365,000 | 218,000 | 347,000 | 327,000 | 275,000 | 232,000 |
| Thrombin time (s) | 10.8 | 9.6 | 10.0 | 10.9 | 13.6 | 16.4 |
| Prothrombin time | 30.8 | 30.7 | 22.6 | 21.5 | 24.7 | 23.0 |
| Aspartate aminotransferase (U/L) | 28 | 48 | 28 | 22 | 10 | 28 |
| Alanine aminotransferase (U/L) | 8 | 24 | 8 | 14 | 10 | 56 |
| Creatinine (mg/dl) | 0.52 | 1.14 | 0.53 | 0.6 | 0.49 | 0.64 |
| Fibrinogen (mg/dL) | 681 | 485 | 856 | 479 | 498 | 601 |
| D-dimer (ng/mL) | 1267 | 1346 | 1739 | 3500 | 5993 | 4716 |
| Procalcitonin (ng/mL) | 0.12 | 1.72 | 0.28 | 1.18 | 0.02 | 0.05 |
| Rx or CAT (COVID signs) | + | + | + | + | + | + |
| Orotracheal intubation | + | − | + | − | − | − |
| Supplemental O2 | − | + | − | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Pliego, A.; Miranda, J.; Vega-Torreblanca, S.; Valdespino-Vázquez, Y.; Helguera-Repetto, C.; Espejel-Nuñez, A.; Borboa-Olivares, H.; Espino y Sosa, S.; Mateu-Rogell, P.; León-Juárez, M.; et al. Molecular Insights into the Thrombotic and Microvascular Injury in Placental Endothelium of Women with Mild or Severe COVID-19. Cells 2021, 10, 364. https://doi.org/10.3390/cells10020364
Flores-Pliego A, Miranda J, Vega-Torreblanca S, Valdespino-Vázquez Y, Helguera-Repetto C, Espejel-Nuñez A, Borboa-Olivares H, Espino y Sosa S, Mateu-Rogell P, León-Juárez M, et al. Molecular Insights into the Thrombotic and Microvascular Injury in Placental Endothelium of Women with Mild or Severe COVID-19. Cells. 2021; 10(2):364. https://doi.org/10.3390/cells10020364
Chicago/Turabian StyleFlores-Pliego, Arturo, Jael Miranda, Sara Vega-Torreblanca, Yolotzin Valdespino-Vázquez, Cecilia Helguera-Repetto, Aurora Espejel-Nuñez, Héctor Borboa-Olivares, Salvador Espino y Sosa, Paloma Mateu-Rogell, Moisés León-Juárez, and et al. 2021. "Molecular Insights into the Thrombotic and Microvascular Injury in Placental Endothelium of Women with Mild or Severe COVID-19" Cells 10, no. 2: 364. https://doi.org/10.3390/cells10020364