The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment
Abstract
:1. Introduction
2. The Crosstalk of the CXCR3 Variants and Their Chemokine Ligands Within the Tumor Microenvironment
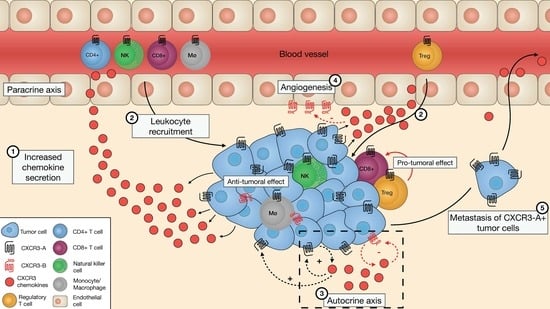
2.1. CXCR3-A on Leukocytes Mediates their Migration to the TME to Modulate Tumor Progression
2.2. CXCR3-A on Malignant Cells in the TME Contributes to Tumor Growth and Dissemination
2.3. CXCR3-B Has an Anti-Proliferative Effect in the Tumor Microenvironment
2.4. Is the CXCR3-B Variant Involved in Angiogenesis Regulation?
2.5. The CXCR3 Ligands are Upregulated in the TME and Act in an Autocrine and Paracrine Manner
2.5.1. CXCL11
2.5.2. CXCL10
2.5.3. CXCL9
2.5.4. CXCL4 and its CXCL4L1 Variant
3. Technical Hurdles to Studying the CXCR3 Variants
4. Conclusions, Challenges, and Future Directions
Funding
Conflicts of Interest
References
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M. Dancing to the tune of chemokines. Nat. Immunol. 2001, 2, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kleist, A.B.; Getschman, A.E.; Ziarek, J.J.; Nevins, A.M.; Gauthier, P.-A.; Chevigné, A.; Szpakowska, M.; Volkman, B.F. New paradigms in chemokine receptor signal transduction: Moving beyond the two-site model. Biochem. Pharmacol. 2016, 114, 53–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpakowska, M.; Dupuis, N.; Baragli, A.; Counson, M.; Hanson, J.; Piette, J.; Chevigné, A. Human herpesvirus 8-encoded chemokine vCCL2/vMIP-II is an agonist of the atypical chemokine receptor ACKR3/CXCR7. Biochem. Pharmacol. 2016, 114, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Alagesan, P.; Desai, N.K.; Pack, T.F.; Wu, J.-H.; Inoue, A.; Freedman, N.J.; Rajagopal, S. CXC motif chemokine receptor 3 splice variants differentially activate beta-arrestins to regulate downstream signaling pathways. Mol. Pharmacol. 2017, 92, 136–150. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front. Immunol. 2018, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Loetscher, P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001, 2, 123–128. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Thelen, M.; Stein, J.V. How chemokines invite leukocytes to dance. Nat. Immunol. 2008, 9, 953–959. [Google Scholar] [CrossRef]
- Luster, A.D. Chemokines—Chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998, 338, 436–445. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relation, T.; Dominici, M.; Horwitz, E.M. Concise review: An (Im) Penetrable shield: How the tumor microenvironment protects cancer stem cells. Stem Cells 2017, 35, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadière, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Groom, J.R.; Luster, A.D. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, K.; Van den Steen, P.E.; Liekens, S.; Van Damme, J.; Struyf, S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015, 26, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Berchiche, Y.A.; Sakmar, T.P. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol. Pharmacol. 2016, 90, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, M.; Gerber, B.; Loetscher, P.; Jones, S.A.; Piali, L.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Chemokine receptor specific for IP10 and mig: Structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 1996, 184, 963–969. [Google Scholar] [CrossRef]
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E. Interferon–inducible T cell alpha chemoattractant (I-TAC): A novel Non-ELR CXC Chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998, 187, 2009–2021. [Google Scholar] [CrossRef]
- Loetscher, M.; Loetscher, P.; Brass, N.; Meese, E.; Moser, B. Lymphocyte-specific chemokine receptor CXCR3: Regulation, chemokine binding and gene localization. Eur. J. Immunol. 1998, 28, 3696–3705. [Google Scholar] [CrossRef]
- Thompson, B.D.; Jin, Y.; Wu, K.H.; Colvin, R.A.; Luster, A.D.; Birnbaumer, L.; Wu, M.X. Inhibition of Gai2 activation by Gai3 in CXCR3-mediated signaling. J. Biol. Chem. 2007, 282, 9547–9555. [Google Scholar] [CrossRef]
- Smit, M.J.; Verdijk, P.; van der Raaij-Helmer, E.M.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi-and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef]
- Mueller, A.; Meiser, A.; McDonagh, E.M.; Fox, J.M.; Petit, S.J.; Xanthou, G.; Williams, T.J.; Pease, J.E. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J. Leukoc. Biol. 2008, 83, 875–882. [Google Scholar] [CrossRef]
- Korniejewska, A.; McKnight, A.J.; Johnson, Z.; Watson, M.L.; Ward, S.G. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 2011, 132, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.O.; Scholten, D.J.; Heitman, L.H.; Vischer, H.F.; Leurs, R. Label-free impedance responses of endogenous and synthetic chemokine receptor CXCR3 agonists correlate with G i-protein pathway activation. Biochem. Biophys. Res. Commun. 2012, 419, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Beltrame, C.; Annunziato, F.; Lasagni, L.; Luconi, M.; Galli, G.; Cosmi, L.; Maggi, E.; Salvadori, M.; Pupilli, C. Role for interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J. Am. Soc. Nephrol. 1999, 10, 2518–2526. [Google Scholar] [PubMed]
- Bonacchi, A.; Romagnani, P.; Romanelli, R.G.; Efsen, E.; Annunziato, F.; Lasagni, L.; Francalanci, M.; Serio, M.; Laffi, G.; Pinzani, M. Signal transduction by the chemokine receptor CXCR3: Activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J. Biol. Chem. 2001, 276, 9945–9954. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Wu, H.-L.; Boyé, K.; Pan, C.-Y.; Chen, Y.-C.; Pujol, N.; Lin, C.-W.; Chiu, L.-Y.; Billottet, C.; Alves, I.D. Oligomerization state of CXCL4 chemokines regulates G protein-coupled receptor activation. Acs Chem. Biol. 2017, 12, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Quemener, C.; Bikfalvi, A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2013, 1836, 287–295. [Google Scholar] [CrossRef]
- Strieter, R.M.; Burdick, M.D.; Gomperts, B.N.; Belperio, J.A.; Keane, M.P. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 593–609. [Google Scholar] [CrossRef] [Green Version]
- Mgrditchian, T.; Arakelian, T.; Paggetti, J.; Noman, M.Z.; Viry, E.; Moussay, E.; Van Moer, K.; Kreis, S.; Guerin, C.; Buart, S. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, E9271–E9279. [Google Scholar] [CrossRef] [Green Version]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- Luster, A.D.; Leder, P. IP-10, a-CXC-chemokine, elicits a potent thymus-dependent antitumor response in vivo. J. Exp. Med. 1993, 178, 1057–1065. [Google Scholar] [CrossRef]
- Mullins, I.M.; Slingluff, C.L.; Lee, J.K.; Garbee, C.F.; Shu, J.; Anderson, S.G.; Mayer, M.E.; Knaus, W.A.; Mullins, D.W. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004, 64, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Hensbergen, P.J.; Wijnands, P.G.B.; Schreurs, M.W.; Scheper, R.J.; Willemze, R.; Tensen, C.P. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J. Immunother. 2005, 28, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Burdick, M.D.; Belperio, J.A.; Xue, Y.Y.; Gerard, C.; Sharma, S.; Dubinett, S.M.; Strieter, R.M. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J. Immunol. 2006, 176, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Reckamp, K.L.; Figlin, R.A.; Moldawer, N.; Pantuck, A.J.; Belldegrun, A.S.; Burdick, M.D.; Strieter, R.M. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy. J. Immunother. 2007, 30, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, M.; Fisher, D.; Matsuzaki, J.; Skitzki, J.; Gaulin, N.; Muhitch, J.; Ku, A.; Frelinger, J.; Odunsi, K.; Gajewski, T. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015, 6, 7458. [Google Scholar] [CrossRef] [PubMed]
- Chheda, Z.S.; Sharma, R.K.; Jala, V.R.; Luster, A.D.; Haribabu, B. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J. Immunol. 2016, 197, 2016–2026. [Google Scholar] [CrossRef]
- Oghumu, S.; Varikuti, S.; Terrazas, C.; Kotov, D.; Nasser, M.W.; Powell, C.A.; Ganju, R.K.; Satoskar, A.R. CXCR 3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology 2014, 143, 109–119. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Luo, J.; Fang, J.; Zhou, H.; Hu, M.; Maskey, N.; Yang, G. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14725. [Google Scholar]
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Cancer Res. 2008, 68, 8437–8445. [Google Scholar] [CrossRef]
- Wenzel, J.; Bekisch, B.; Uerlich, M.; Haller, O.; Bieber, T.; Tüting, T. Type I interferon–associated recruitment of cytotoxic lymphocytes: A common mechanism in regressive melanocytic lesions. Am. J. Clin. Pathol. 2005, 124, 37–48. [Google Scholar] [CrossRef]
- Redjimi, N.; Raffin, C.; Raimbaud, I.; Pignon, P.; Matsuzaki, J.; Odunsi, K.; Valmori, D.; Ayyoub, M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012, 72, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Ling, C.C.; Shao, Y.; Xu, A.; Li, X.C.; Ng, K.T.-P.; Liu, X.B.; Ma, Y.Y.; Qi, X.; Liu, H. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J. Hepatol. 2016, 65, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, A.E.; Brotman, J.J.; Pittman, M.E.; Judd, N.P.; Lewis, J.S.; Schreiber, R.D.; Uppaluri, R. CXCR3 enhances a T cell dependent epidermal proliferative response and promotes skin tumorigenesis. Cancer Res. 2011, 71, 5707–5716. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Haensch, W.; Röefzaad, C.; Schlag, P.M. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001, 61, 3932–3936. [Google Scholar] [PubMed]
- Sackstein, R.; Schatton, T.; Barthel, S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Investig. 2017, 97, 669–697. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Jenkins, M.K.; Azuma, M.; Fife, B.T. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 2013, 62, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Maru, S.V.; Holloway, K.A.; Flynn, G.; Lancashire, C.L.; Loughlin, A.J.; Male, D.K.; Romero, I.A. Chemokine production and chemokine receptor expression by human glioma cells: Role of CXCL10 in tumour cell proliferation. J. Neuroimmunol. 2008, 199, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Bonomini, S.; Romagnani, P.; Lazzaretti, M.; Morandi, F.; Colla, S.; Tagliaferri, S.; Lasagni, L.; Annunziato, F.; Crugnola, M. CXCR3 and its binding chemokines in myeloma cells: Expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica 2006, 91, 1489–1497. [Google Scholar]
- Bai, M.; Chen, X.; Ba, Y. CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer. Mol. Clin. Oncol. 2016, 4, 23–30. [Google Scholar] [CrossRef]
- Urra, S.; Fischer, M.C.; Martínez, J.R.; Véliz, L.; Orellana, P.; Solar, A.; Bohmwald, K.; Kalergis, A.; Riedel, C.; Corvalán, A.H. Differential expression profile of CXCR3 splicing variants is associated with thyroid neoplasia. Potential role in papillary thyroid carcinoma oncogenesis? Oncotarget 2018, 9, 2445–2467. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; Leppert, J.T.; Riggs, S.B.; Yu, H.; Zomorodian, N.; Kabbinavar, F.F.; Strieter, R.M.; Belldegrun, A.S.; Pantuck, A.J. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J. Urol. 2008, 179, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Sonoshita, M.; Sakashita, H.; Takabayashi, A.; Yamaoka, Y.; Manabe, T.; Inaba, K.; Minato, N.; Oshima, M.; Taketo, M.M. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004, 64, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, C.; Martin, J.M.; Jorda, E.; Llombart-Bosch, A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: Correlation with clinicopathological prognostic factors. J. Clin. Pathol. 2007, 60, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Momtahen, S.; Lee, B.A.; Swanson, D.L.; Pavlovic, M.D. Epidermotropic B-cell lymphoma: A unique subset of CXCR3-positive marginal zone lymphoma. Am. J. Dermatopathol. 2016, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Trentin, L.; Agostini, C.; Facco, M.; Piazza, F.; Perin, A.; Siviero, M.; Gurrieri, C.; Galvan, S.; Adami, F.; Zambello, R. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J. Clin. Investig. 1999, 104, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Luo, D.; Reynolds, B.A.; Meher, G.; Katritzky, A.R.; Lu, B.; Gerard, C.J.; Bhadha, C.P.; Harrison, J.K. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis 2010, 32, 129–137. [Google Scholar] [CrossRef]
- Pu, Y.; Li, S.; Zhang, C.; Bao, Z.; Yang, Z.; Sun, L. High expression of CXCR3 is an independent prognostic factor in glioblastoma patients that promotes an invasive phenotype. J. Neuro-Oncol. 2015, 122, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kawada, K.; Iwamoto, M.; Akagami, M.; Hida, K.; Nakanishi, Y.; Kanda, K.; Kawada, M.; Seno, H.; Taketo, M.M. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int. J. Cancer 2013, 132, 276–287. [Google Scholar] [CrossRef]
- Kawada, K.; Hosogi, H.; Sonoshita, M.; Sakashita, H.; Manabe, T.; Shimahara, Y.; Sakai, Y.; Takabayashi, A.; Oshima, M.; Taketo, M. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 2007, 26, 4679–4688. [Google Scholar] [CrossRef]
- Windmüller, C.; Zech, D.; Avril, S.; Boxberg, M.; Dawidek, T.; Schmalfeldt, B.; Schmitt, M.; Kiechle, M.; Bronger, H. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis 2017, 6, e331. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Rabbe, N.; Antoine, M.; Vieira, T.; Poulot, V.; Cadranel, J.; Wislez, M. Pro-tumoural CXCL10/CXCR3-A autocrine loop in invasive mucinous lung adenocarcinoma. ERJ Open Res. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Norsworthy, K.; Kundu, N.; Rodgers, W.H.; Gimotty, P.A.; Goloubeva, O.; Lipsky, M.; Li, Y.; Holt, D.; Fulton, A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol. Cancer Ther. 2009, 8, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Yan, H.H.; Pang, Y.; Jian, J.; Achyut, B.R.; Liang, X.; Weiss, J.M.; Wiltrout, R.H.; Hollander, M.C.; Yang, L. CXCR3 as a molecular target in breast cancer metastasis: Inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget 2015, 6, 43408–43419. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Maggi, L.; Mazzinghi, B.; Cosmi, L.; Lasagni, L.; Liotta, F.; Lazzeri, E.; Angeli, R.; Rotondi, M.; Filì, L. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J. Allergy Clin. Immunol. 2005, 116, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, S.G.; Aksoy, M.O.; Yang, Y.; Shahabuddin, S.; Litvin, J.; Safadi, F.; Rogers, T.J. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L584–L591. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J.; Wells, A. Differential regulation of pericyte function by the CXC receptor 3. Wound Repair Regen. 2015, 23, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Monk, P.N.; Finn, A. Cxc chemokine receptor expression on human endothelial cells. Cytokine 1999, 11, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Contreras, A.G.; Grimm, M.; Waaga-Gasser, A.M.; Briscoe, D.M.; Pal, S. Calcineurin inhibitors modulate CXCR3 splice variant expression and mediate renal cancer progression. J. Am. Soc. Nephrol. 2008, 19, 2437–2446. [Google Scholar] [CrossRef]
- Datta, D.; Banerjee, P.; Gasser, M.; Waaga-Gasser, A.M.; Pal, S. CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J. Biol. Chem. 2010, 285, 36842–36848. [Google Scholar] [CrossRef]
- Furuya, M.; Yoneyama, T.; Miyagi, E.; Tanaka, R.; Nagahama, K.; Miyagi, Y.; Nagashima, Y.; Hirahara, F.; Inayama, Y.; Aoki, I. Differential expression patterns of CXCR3 variants and corresponding CXC chemokines in clear cell ovarian cancers and endometriosis. Gynecol. Oncol. 2011, 122, 648–655. [Google Scholar] [CrossRef]
- Balan, M.; Pal, S. A novel CXCR3-B chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation. J. Biol. Chem. 2014, 289, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Flaxenburg, J.A.; Laxmanan, S.; Geehan, C.; Grimm, M.; Waaga-Gasser, A.M.; Briscoe, D.M.; Pal, S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: Relevance for the development of human breast cancer. Cancer Res. 2006, 66, 9509–9518. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Serni, S.; Lapini, A.; Vittori, G.; Alessandrini, M.; Nesi, G.; Palli, D.; Carini, M. CXCR3-B expression correlates with tumor necrosis extension in renal cell carcinoma. J. Urol. 2009, 181, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Reader, J.C.; Ma, X.; Kundu, N.; Kochel, T.; Fulton, A.M. Divergent roles of CXCR3 isoforms in promoting cancer stem-like cell survival and metastasis. Breast Cancer Res. Treat. 2015, 149, 403–415. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Angiogenesis: Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Maione, T.E.; Gray, G.S.; Petro, J.; Hunt, A.J.; Donner, A.L.; Bauer, S.I.; Carson, H.F.; Sharpe, R.J. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 1990, 247, 77–79. [Google Scholar] [CrossRef]
- Luster, A.D.; Greenberg, S.M.; Leder, P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J. Exp. Med. 1995, 182, 219–231. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef]
- Arenberg, D.A.; Kunkel, S.L.; Polverini, P.J.; Morris, S.B.; Burdick, M.D.; Glass, M.C.; Taub, D.T.; Iannettoni, M.D.; Whyte, R.I.; Strieter, R.M. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 1996, 184, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Rupertus, K.; Sinistra, J.; Scheuer, C.; Nickels, R.M.; Schilling, M.K.; Menger, M.D.; Kollmar, O. Interaction of the chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of tumor angiogenesis of colorectal cancer. Clin. Exp. Metastasis 2014, 31, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Campanella, G.S.; Colvin, R.A.; Luster, A.D. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 2010, 5, e12700. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Peng, D.; Kryczek, I.; Wu, K.; Li, W.; Zhao, E.; Zhao, L.; Wei, S.; Frankel, T.; Vatan, L. PRC2 epigenetically silences Th1-type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 2016, 76, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bronger, H.; Singer, J.; Windmüller, C.; Reuning, U.; Zech, D.; Delbridge, C.; Dorn, J.; Kiechle, M.; Schmalfeldt, B.; Schmitt, M.; et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer 2016, 115, 553–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronger, H.; Kraeft, S.; Schwarz-Boeger, U.; Cerny, C.; Stöckel, A.; Avril, S.; Kiechle, M.; Schmitt, M. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 2012, 14, R30. [Google Scholar] [CrossRef]
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008, 13, 1634–1645. [Google Scholar] [CrossRef]
- Mortier, A.; Van Damme, J.; Proost, P. Overview of the mechanisms regulating chemokine activity and availability. Immunol. Lett. 2012, 145, 2–9. [Google Scholar] [CrossRef]
- Proost, P.; Schutyser, E.; Menten, P.; Struyf, S.; Wuyts, A.; Opdenakker, G.; Detheux, M.; Parmentier, M.; Durinx, C.; Lambeir, A.-M. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 2001, 98, 3554–3561. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.B.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef]
- Karin, N.; Wildbaum, G.; Thelen, M. Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J. Leukoc. Biol. 2016, 99, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Z.; Xu, L.; Che, X.; Wen, T.; Fan, Y.; Li, C.; Wang, S.; Cheng, Y.; Wang, X. CXCL9/10/11, a regulator of PD-L1 expression in gastric cancer. BMC Cancer 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Szpakowska, M.; Nevins, A.M.; Meyrath, M.; Rhainds, D.; D’huys, T.; Guité-Vinet, F.; Dupuis, N.; Gauthier, P.A.; Counson, M.; Kleist, A. Different contributions of chemokine N-terminal features attest to a different ligand binding mode and a bias towards activation of ACKR3/CXCR7 compared with CXCR4 and CXCR3. Br. J. Pharmacol. 2018, 175, 1419–1438. [Google Scholar] [CrossRef] [PubMed]
- Sierro, F.; Biben, C.; Martínez-Muñoz, L.; Mellado, M.; Ransohoff, R.M.; Li, M.; Woehl, B.; Leung, H.; Groom, J.; Batten, M. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc. Natl. Acad. Sci. USA 2007, 104, 14759–14764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zohar, Y.; Wildbaum, G.; Novak, R.; Salzman, A.L.; Thelen, M.; Alon, R.; Barsheshet, Y.; Karp, C.L.; Karin, N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J. Clin. Investig. 2014, 124, 2009–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heise, C.E.; Pahuja, A.; Hudson, S.C.; Mistry, M.S.; Putnam, A.L.; Gross, M.M.; Gottlieb, P.A.; Wade, W.S.; Kiankarimi, M.; Schwarz, D. Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Verpoest, S.; Van de Borne, K.; Schutyser, E.; Struyf, S.; Put, W.; Ronsse, I.; Grillet, B.; Opdenakker, G.; Van Damme, J. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-γ in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. J. Leukoc. Biol. 2004, 75, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Sauty, A.; Colvin, R.A.; Wagner, L.; Rochat, S.; Spertini, F.; Luster, A.D. CXCR3 internalization following T cell-endothelial cell contact: Preferential role of IFN-inducible T cell α chemoattractant (CXCL11). J. Immunol. 2001, 167, 7084–7093. [Google Scholar] [CrossRef] [PubMed]
- Szpakowska, M.; Meyrath, M.; Reynders, N.; Counson, M.; Hanson, J.; Steyaert, J.; Chevigné, A. Mutational analysis of the extracellular disulphide bridges of the atypical chemokine receptor ACKR3/CXCR7 uncovers multiple binding and activation modes for its chemokine and endogenous non-chemokine agonists. Biochem. Pharmacol. 2018, 153, 299–309. [Google Scholar] [CrossRef]
- Burns, J.M.; Summers, B.C.; Wang, Y.; Melikian, A.; Berahovich, R.; Miao, Z.; Penfold, M.E.; Sunshine, M.J.; Littman, D.R.; Kuo, C.J. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2006, 203, 2201–2213. [Google Scholar] [CrossRef]
- Massara, M.; Bonavita, O.; Mantovani, A.; Locati, M.; Bonecchi, R. Atypical chemokine receptors in cancer: Friends or foes? J. Leukoc. Biol. 2016, 99, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Desnoyer, A.; Meuris, F.; Bachelerie, F.; Balabanian, K.; Machelon, V. The relevance of the chemokine receptor ACKR3/CXCR7 on CXCL12-mediated effects in cancers with a focus on virus-related cancers. Cytokine Growth Factor Rev. 2014, 25, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; De Lin Liu, S.L.; Yuan, G.F.; Li, L.; Zhu, H.Y.; Cao, G.Y. Down-regulation of cXcl11 inhibits colorectal cancer cell growth and epithelial-mesenchymal transition. Oncotargets Ther. 2018, 11, 7333–7343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Li, S.; Lv, M.; Yang, X.; Li, M.; Zhang, Z. CXCL11 promotes self-renewal and tumorigenicity of α2δ1+ liver tumor-initiating cells through CXCR3/ERK1/2 signaling. Cancer Lett. 2019, 449, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.M.; Raitman, I.; Feeley, L.; Pinnaduwage, D.; Nguyen, L.T.; O’Malley, F.P.; Ohashi, P.S.; Andrulis, I.L. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. 2013, 19, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Fujikawa, H.; Kawamura, M.; Matsushita, K.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Uchida, K.; Mohri, Y.; Kusunoki, M. Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int. J. Oncol. 2012, 40, 560–566. [Google Scholar] [CrossRef]
- Zipin-Roitman, A.; Meshel, T.; Sagi-Assif, O.; Shalmon, B.; Avivi, C.; Pfeffer, R.M.; Witz, I.P.; Ben-Baruch, A. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007, 67, 3396–3405. [Google Scholar] [CrossRef]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2014, 6, 2981–2991. [Google Scholar] [CrossRef]
- Sato, Y.; Motoyama, S.; Nanjo, H.; Wakita, A.; Yoshino, K.; Sasaki, T.; Nagaki, Y.; Liu, J.; Imai, K.; Saito, H. CXCL10 expression status is prognostic in patients with advanced thoracic esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2016, 23, 936–942. [Google Scholar] [CrossRef]
- Wightman, S.; Uppal, A.; Pitroda, S.; Ganai, S.; Burnette, B.; Stack, M.; Oshima, G.; Khan, S.; Huang, X.; Posner, M. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br. J. Cancer 2015, 113, 327–335. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Han, X.; Li, Z.; Zhu, Q.; Yan, J.; Yu, S.; Jin, Z.; Wang, Z.; Zheng, Q. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed. Pharmacother. 2016, 78, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, H.; Wang, Z.; Zhang, G. The Inflammatory CXC Chemokines, GROαhigh, IP-10low, and MIGlow, in Tumor Microenvironment Can Be Used as New Indicators for Non-small Cell Lung Cancer Progression. Immunol. Investig. 2017, 46, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Gorbachev, A.V.; Kobayashi, H.; Kudo, D.; Tannenbaum, C.S.; Finke, J.H.; Shu, S.; Farber, J.M.; Fairchild, R.L. CXC chemokine ligand 9/monokine induced by IFN-γ production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J. Immunol. 2007, 178, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, H.; Jin, Z.; Takegawa, S.; Nakayama, T.; Yoshie, O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2009, 217, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sgadari, C.; Farber, J.M.; Angiolillo, A.L.; Liao, F.; Teruya-Feldstein, J.; Burd, P.R.; Yao, L.; Gupta, G.; Kanegane, C.; Tosato, G. Mig, the monokine induced by interferon-γ, promotes tumor necrosis in vivo. Blood 1997, 89, 2635–2643. [Google Scholar] [PubMed]
- Amatschek, S.; Lucas, R.; Eger, A.; Pflueger, M.; Hundsberger, H.; Knoll, C.; Grosse-Kracht, S.; Schuett, W.; Koszik, F.; Maurer, D. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br. J. Cancer 2011, 104, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Li, L.; Shao, S.; Wu, J.; Bian, L.; He, Y. Epithelial mesenchymal transition induced by the CXCL9/CXCR3 axis through AKT activation promotes invasion and metastasis in tongue squamous cell carcinoma. Oncol. Rep. 2018, 39, 1356–1368. [Google Scholar] [CrossRef]
- Mir, M.A.; Maurer, M.J.; Ziesmer, S.C.; Slager, S.L.; Habermann, T.; Macon, W.R.; Link, B.K.; Syrbu, S.; Witzig, T.; Friedberg, J.W. Elevated serum levels of IL-2R, IL-1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood 2015, 125, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Pucci, F.; Rickelt, S.; Newton, A.P.; Garris, C.; Nunes, E.; Evavold, C.; Pfirschke, C.; Engblom, C.; Mino-Kenudson, M.; Hynes, R.O. PF4 promotes platelet production and lung cancer growth. Cell Rep. 2016, 17, 1764–1772. [Google Scholar] [CrossRef]
- Deng, S.; Deng, Q.; Zhang, Y.; Ye, H.; Yu, X.; Zhang, Y.; Han, G.Y.; Luo, P.; Wu, M.; Yu, Y. Non-platelet-derived CXCL4 differentially regulates cytotoxic and regulatory T cells through CXCR3 to suppress the immune response to colon cancer. Cancer Lett. 2019, 443, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Wang, X.; Deng, S.; Ye, H.; Guan, W.; Wu, M.; Zhu, S.; Yu, Y.; Han, W. CXCL4 mediates tumor regrowth after chemotherapy by suppression of antitumor immunity. Cancer Biol. Ther. 2015, 16, 1775–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struyf, S.; Salogni, L.; Burdick, M.D.; Vandercappellen, J.; Gouwy, M.; Noppen, S.; Proost, P.; Opdenakker, G.; Parmentier, M.; Gerard, C. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood 2011, 117, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struyf, S.; Burdick, M.D.; Proost, P.; Van Damme, J.; Strieter, R.M. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ. Res. 2004, 95, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Struyf, S.; Burdick, M.D.; Peeters, E.; Van den Broeck, K.; Dillen, C.; Proost, P.; Van Damme, J.; Strieter, R.M. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growth and metastasis by preventing angiogenesis. Cancer Res. 2007, 67, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Quemener, C.; Baud, J.; Boyé, K.; Dubrac, A.; Billottet, C.; Soulet, F.; Darlot, F.; Dumartin, L.; Sire, M.; Grepin, R. Dual roles for CXCL4 chemokines and CXCR3 in angiogenesis and invasion of pancreatic cancer. Cancer Res. 2016, 76, 6507–6519. [Google Scholar] [CrossRef]
- Karjalainen, M.K.; Ojaniemi, M.; Haapalainen, A.M.; Mahlman, M.; Salminen, A.; Huusko, J.M.; Määttä, T.A.; Kaukola, T.; Anttonen, J.; Ulvila, J. CXCR3 polymorphism and expression associate with spontaneous preterm birth. J. Immunol. 2015, 195, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dhir, R.; Wells, A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol. Cancer 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Baribaud, F.; Edwards, T.G.; Sharron, M.; Brelot, A.; Heveker, N.; Price, K.; Mortari, F.; Alizon, M.; Tsang, M.; Doms, R.W. Antigenically distinct conformations of CXCR4. J. Virol. 2001, 75, 8957–8967. [Google Scholar] [CrossRef]
- Blanpain, C.; Vanderwinden, J.-M.; Cihak, J.; Wittamer, V.; Le Poul, E.; Issafras, H.; Stangassinger, M.; Vassart, G.; Marullo, S.; Schlöndorff, D.; et al. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol. Biol. Cell 2002, 13, 723–737. [Google Scholar] [CrossRef]
- Jopling, L.; Watt, G.; Fisher, S.; Birch, H.; Coggon, S.; Christie, M. Analysis of the pharmacokinetic/pharmacodynamic relationship of a small molecule CXCR3 antagonist, NBI-74330, using a murine CXCR3 internalization assay. Br. J. Pharmacol. 2007, 152, 1260–1271. [Google Scholar] [CrossRef] [Green Version]
- Scholten, D.; Canals, M.; Wijtmans, M.; De Munnik, S.; Nguyen, P.; Verzijl, D.; De Esch, I.; Vischer, H.; Smit, M.; Leurs, R. Pharmacological characterization of a small-molecule agonist for the chemokine receptor CXCR3. Br. J. Pharmacol. 2012, 166, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.P.; Cox, R.J. Small molecule CXCR3 antagonists. J. Med. Chem. 2015, 59, 2894–2917. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynders, N.; Abboud, D.; Baragli, A.; Noman, M.Z.; Rogister, B.; Niclou, S.P.; Heveker, N.; Janji, B.; Hanson, J.; Szpakowska, M.; et al. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment. Cells 2019, 8, 613. https://doi.org/10.3390/cells8060613
Reynders N, Abboud D, Baragli A, Noman MZ, Rogister B, Niclou SP, Heveker N, Janji B, Hanson J, Szpakowska M, et al. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment. Cells. 2019; 8(6):613. https://doi.org/10.3390/cells8060613
Chicago/Turabian StyleReynders, Nathan, Dayana Abboud, Alessandra Baragli, Muhammad Zaeem Noman, Bernard Rogister, Simone P. Niclou, Nikolaus Heveker, Bassam Janji, Julien Hanson, Martyna Szpakowska, and et al. 2019. "The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment" Cells 8, no. 6: 613. https://doi.org/10.3390/cells8060613