Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice
Abstract
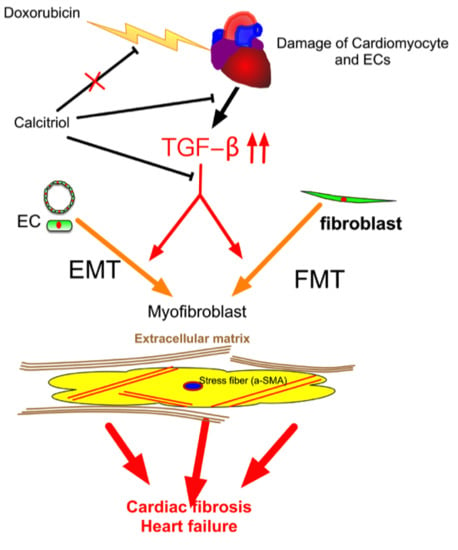
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of Doxorubicin-Induction Cardiomyopathy (DoIC)
2.3. Induction of GFP in Tek-Expressing Cells
2.4. Cell Culture
2.5. Histology and Immunofluorescent Staining
2.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Western Blot Analysis
2.8. Statistics
3. Results
3.1. Calcitriol Attenuated Doxorubicin-Induced Impariment of Cardiac Diastolic Function
3.2. Calcitriol Reduced Dox-Induced Heart Failure Markers but did not Prevent Dox-Reduced Cardiac Sizes
3.3. Calcitriol Attenuated Dox-Induced Cardiac Fibrosis and Pro-Fibrotic Protein Expression
3.4. Calcitriol Attenuated Doxorubicin-Induced EndMT in Mouse Model
3.5. Calcitriol did not Inhibit Dox-Induced DNA Damage of Cardiac Myocytes and Endothelial Cells
3.6. Calcitriol Inhibited Dox-Induced p-Smad2 Activation in Endothelial Cells In Vivo
3.7. Calcitriol Attenuated the EndMT and FMT Process by the Suppression of TGF-β1 in the In Vitro Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| cTnI | Cardiac troponin I |
| Dox | Doxorubicin |
| DoIC | Dox-induced cardiomyopathy |
| ECM | Extracellular matix |
| EndMT | Endothelial-to-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| FMT | Fibroblast-to-myofibroblast transition |
| HW/BW | Heart weight to body weight ratio |
| H&E | Hematoxylin and eosin |
| HUVECs | Human umbilical vein endothelial cells |
| IF | Immunofluorescence |
| IVSd | Interventricular septum thickness at mm end-diastole |
| IVRT | Iso-volemic relaxation time |
| LVPWd | Left ventricular posterior wall thickness at end-diastole |
| LVIDd | Left ventricular internal dimension at end-diastole |
| LVIDs | Left ventricular internal dimension mm at end-systole |
| qRT-PCR | Quantitative reverse transcrition polymerase chain reaction |
| SERCA | Sarcoplasmic/endoplasmic reticulum calcium ATPase |
| TekCreERT2/DRG | Tek-CreERT2;UBC-DsRed-emGFP doulble transgenic mice |
| VEGF | Vascular endothelial growth factor |
| WGA | Wheat germ agglutinin |
References
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugnes, H.S.; Wethal, T.; Aass, N.; Dahl, O.; Klepp, O.; Langberg, C.W.; Wilsgaard, T.; Bremnes, R.M.; Fossa, S.D. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: A 20-year follow-up study. J. Clin. Oncol. 2010, 28, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Prestor, V.V.; Rakovec, P.; Kozelj, M.; Jereb, B. Late cardiac damage of anthracycline therapy for acute lymphoblastic leukemia in childhood. Pediatric Hematol. Oncol. 2000, 17, 527–540. [Google Scholar] [CrossRef]
- Milano, G.; Raucci, A.; Scopece, A.; Daniele, R.; Guerrini, U.; Sironi, L.; Cardinale, D.; Capogrossi, M.C.; Pompilio, G. Doxorubicin and trastuzumab regimen induces biventricular failure in mice. J. Am. Soc. Echocardiogr. 2014, 27, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Dorup, I.; Levitt, G.; Sullivan, I.; Sorensen, K. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: The role of diastolic function. Heart 2004, 90, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.W.; Narahara, K.A.; Ritchie, J.L.; Hamilton, G.W.; Kennedy, J.W. Time- and dose-dependent changes in ejection fraction determined by radionuclide angiography after anthracycline therapy. Cancer Treat. Rep. 1978, 62, 945–948. [Google Scholar]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Jensen, B.V.; Skovsgaard, T.; Nielsen, S.L. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002, 13, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. New Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Hulshoff, M.S.; Xu, X.; Krenning, G.; Zeisberg, E.M. Epigenetic Regulation of Endothelial-to-Mesenchymal Transition in Chronic Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1986–1996. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, B.; Gu, Y.; Groome, L.J.; Sun, J.; Wang, Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 56–62. [Google Scholar] [CrossRef]
- Grundmann, M.; Haidar, M.; Placzko, S.; Niendorf, R.; Darashchonak, N.; Hubel, C.A.; von Versen-Hoynck, F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. 2012, 303, C954–C962. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.K.; Byrom, R.; Gierula, J.; Paton, M.F.; Jamil, H.A.; Lowry, J.E.; Gillott, R.G.; Barnes, S.A.; Chumun, H.; Kearney, L.C.; et al. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J. Am. Coll. Cardiol. 2016, 67, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; MacLennan, G.S.; Avenell, A.; Bolland, M.; Grey, A.; Witham, M.; Group, R.T. Cardiovascular disease and vitamin D supplementation: Trial analysis, systematic review, and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, C.J.; Warner, S.G. A New Role for Vitamin D: The Enhancement of Oncolytic Viral Therapy in Pancreatic Cancer. Biomedicines 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, B.; Taha, M.F.; Javeri, A. Vitamin C counteracts miR-302/367-induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J. Cell Physiol. 2019, 234, 2672–2682. [Google Scholar] [CrossRef]
- Roehlen, N.; Doering, C.; Hansmann, M.L.; Gruenwald, F.; Vorlaender, C.; Bechstein, W.O.; Holzer, K.; Badenhoop, K.; Penna-Martinez, M. Vitamin D, FOXO3a, and Sirtuin1 in Hashimoto’s Thyroiditis and Differentiated Thyroid Cancer. Front. Endocrinol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Gao, Y.; Ta, N.; Zhang, Y.; Cai, J.; Zhao, Y.; Liu, S.; Zheng, J. MEG3 Activated by Vitamin D Inhibits Colorectal Cancer Cells Proliferation and Migration via Regulating Clusterin. EBioMedicine 2018, 30, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Farhad, H.; Staziaki, P.V.; Addison, D.; Coelho-Filho, O.R.; Shah, R.V.; Mitchell, R.N.; Szilveszter, B.; Abbasi, S.A.; Kwong, R.Y.; Scherrer-Crosbie, M.; et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated With Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [Green Version]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Grabner, A.; Hermann, L.; Richter, B.; Schmitz, K.; Fischer, D.C.; Yanucil, C.; Faul, C.; Haffner, D. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol. Dial. Transplant. 2017, 32, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Reuter, S.; Field, L.J. Targeted expression of cyclin D2 ameliorates late stage anthracycline cardiotoxicity. Cardiovasc. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bassani, J.W.; Bassani, R.A. SERCA upregulation: Breaking the positive feedback in heart failure? Cardiovasc. Res. 2005, 67, 581–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-beta-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar]
- Reed, A.L.; Tanaka, A.; Sorescu, D.; Liu, H.; Jeong, E.M.; Sturdy, M.; Walp, E.R.; Dudley, S.C., Jr.; Sutliff, R.L. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H824–H831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellims, A.H.; Iles, L.M.; Ling, L.H.; Hare, J.L.; Kaye, D.M.; Taylor, A.J. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J. Cardiovasc. Magn. Reson. 2012, 14, 76. [Google Scholar] [CrossRef]
- Hussain, T.; Dragulescu, A.; Benson, L.; Yoo, S.J.; Meng, H.; Windram, J.; Wong, D.; Greiser, A.; Friedberg, M.; Mertens, L.; et al. Quantification and significance of diffuse myocardial fibrosis and diastolic dysfunction in childhood hypertrophic cardiomyopathy. Pediatric Cardiol. 2015, 36, 970–978. [Google Scholar] [CrossRef]
- Niss, O.; Fleck, R.; Makue, F.; Alsaied, T.; Desai, P.; Towbin, J.A.; Malik, P.; Taylor, M.D.; Quinn, C.T. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood 2017, 130, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Su, M.Y.; Lin, L.Y.; Tseng, Y.H.; Chang, C.C.; Wu, C.K.; Lin, J.L.; Tseng, W.Y. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovasc. Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef]
- Doroshow, J.H.; Tallent, C.; Schechter, J.E. Ultrastructural features of Adriamycin-induced skeletal and cardiac muscle toxicity. Am. J. Pathol. 1985, 118, 288–297. [Google Scholar] [PubMed]
- Bernaba, B.N.; Chan, J.B.; Lai, C.K.; Fishbein, M.C. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc. Pathol. 2010, 19, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Nousiainen, T.; Vanninen, E.; Jantunen, E.; Puustinen, J.; Remes, J.; Rantala, A.; Vuolteenaho, O.; Hartikainen, J. Natriuretic peptides during the development of doxorubicin-induced left ventricular diastolic dysfunction. J. Intern. Med. 2002, 251, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Garg, A.; Avramopoulos, P.; Engelhardt, S.; Streckfuss-Bomeke, K.; Batkai, S.; Thum, T. miR-212/132 Cluster Modulation Prevents Doxorubicin-Mediated Atrophy and Cardiotoxicity. Mol. Ther. 2019, 27, 17–28. [Google Scholar] [CrossRef]
- Shimauchi, T.; Numaga-Tomita, T.; Ito, T.; Nishimura, A.; Matsukane, R.; Oda, S.; Hoka, S.; Ide, T.; Koitabashi, N.; Uchida, K.; et al. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight 2017, 2, e93358. [Google Scholar]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Wilkinson, E.L.; Sidaway, J.E.; Cross, M.J. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol. Open 2016, 5, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, E.; Ruiz-Ruiz, C.; Quesada, A.J.; Hernandez, G.; Rodriguez, A.; Lopez-Rivas, A.; Redondo, J.M. Doxorubicin induces apoptosis and CD95 gene expression in human primary endothelial cells through a p53-dependent mechanism. J. Biol. Chem. 2002, 277, 10883–10892. [Google Scholar] [CrossRef]
- Shany, S.; Sigal-Batikoff, I.; Lamprecht, S. Vitamin D and Myofibroblasts in Fibrosis and Cancer: At Cross-purposes with TGF-beta/SMAD Signaling. Anticancer Res. 2016, 36, 6225–6234. [Google Scholar] [CrossRef]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.; Boroomand, S.; Carthy, J.; Luo, Z.; McManus, B. 1,25 Dihydroxyvitamin D3 Inhibits TGFbeta1-Mediated Primary Human Cardiac Myofibroblast Activation. PLoS ONE 2015, 10, e0128655. [Google Scholar] [CrossRef]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; He, W.; Liu, Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int. 2009, 76, 1248–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrilees, M.J.; Sodek, J. Synthesis of TGF-beta 1 by vascular endothelial cells is correlated with cell spreading. J. Vasc. Res. 1992, 29, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Rawal, P.; Siddiqui, H.; Hassan, M.; Choudhary, M.C.; Tripathi, D.M.; Nain, V.; Trehanpati, N.; Kaur, S. Endothelial Cell-Derived TGF-beta Promotes Epithelial-Mesenchymal Transition via CD133 in HBx-Infected Hepatoma Cells. Front. Oncol. 2019, 9, 308. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Jia, L.; Han, Y.; Cheng, J.; Li, H.; Qi, Y.; Du, J. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 2012, 7, e35144. [Google Scholar]
- Gao, L.; Cao, J.T.; Liang, Y.; Zhao, Y.C.; Lin, X.H.; Li, X.C.; Tan, Y.J.; Li, J.Y.; Zhou, C.L.; Xu, H.Y.; et al. Calcitriol attenuates cardiac remodeling and dysfunction in a murine model of polycystic ovary syndrome. Endocrine 2016, 52, 363–373. [Google Scholar] [CrossRef]
- Wong, M.S.; Leisegang, M.S.; Kruse, C.; Vogel, J.; Schurmann, C.; Dehne, N.; Weigert, A.; Herrmann, E.; Brune, B.; Shah, A.M.; et al. Vitamin D promotes vascular regeneration. Circulation 2014, 130, 976–986. [Google Scholar] [CrossRef]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Pechoux, C.; Bogaard, H.J.; Dorfmuller, P.; Remy, S.; Lecerf, F.; Plante, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Maleszewska, M.; Moonen, J.R.; Huijkman, N.; van de Sluis, B.; Krenning, G.; Harmsen, M.C. IL-1beta and TGFbeta2 synergistically induce endothelial to mesenchymal transition in an NFkappaB-dependent manner. Immunobiology 2013, 218, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.B.; Li, T.H.; Huang, C.C.; Tsai, H.C.; Huang, S.F.; Hsieh, Y.C.; Yang, Y.Y.; Huang, Y.H.; Hou, M.C.; Lin, H.C. Chronic calcitriol supplementation improves the inflammatory profiles of circulating monocytes and the associated intestinal/adipose tissue alteration in a diet-induced steatohepatitis rat model. PLoS ONE 2018, 13, e0194867. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chang, J.H.; Chen, C.C.; Su, S.B.; Yang, L.K.; Ma, W.Y.; Zheng, C.M.; Diang, L.K.; Lu, K.C. Calcitriol treatment attenuates inflammation and oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Tohoku J. Exp. Med. 2011, 223, 153–159. [Google Scholar] [CrossRef] [PubMed]








| Ctrl | Dox | Dox–Calcitriol | |
|---|---|---|---|
| Baseline | |||
| LVSd (mm) | 0.78 ± 0.13 | 0.79 ± 0.08 | 0.78 ± 0.11 |
| LVPWd (mm) | 0.63 ± 0.11 | 0.67 ± 0.15 | 0.68± 0.13 |
| LVIDd (mm) | 2.933 ± 0.31 | 2.98 ± 0.17 | 2.89 ± 0.16 |
| LVIDs (mm) | 1.938 ± 0.13 | 2.13 ± 0.18 | 2.13 ± 0.17 |
| LV mass index | 51.16 ± 22 | 53.1 ± 17 | 52.6 ± 23 |
| EF (%) | 66.7 ± 8.1 | 67.2± 7.2 | 66.7 ± 7.6 |
| FS | 35.9 ± 6.3 | 37.6 ± 5.4 | 36.0 ± 7.9 |
| E/E′ | 46 ± 7.8 | 48 ± 6.2 | 45 ± 8.7 |
| IVRT | 15.3± 1.2 | 16.2 ± 1.8 | 15.7 ± 1.5 |
| 12 weeks later | |||
| LVSd (mm) | 0.82 ± 0.15 | 0.74 ± 0.16 * | 0.72 ± 0.03 * |
| LVPWd (mm) | 0.76 ± 0.16 | 0.62 ± 0.17 * | 0.61 ± 0.06 * |
| LVIDd (mm) | 3.3 ± 0.34 | 3.87 ± 0.13 * | 3.78 ± 0.21 * |
| LVIDs (mm) | 2.13 ± 0.12 | 2.88 ± 0.14 * | 2.75 ± 0.18 * |
| LV mass index | 82 ± 19 | 43 ± 16 * | 45 ± 22 * |
| EF (%) | 64 ± 8.1 | 51 ± 7.2 * | 52 ± 5.3 * |
| FS | 35.3 ± 7.2 | 25.2 ± 6.4 * | 26.3 ± 6.2 * |
| E/E′ | 47 ± 3.8 | 87 ± 9.6 * | 67 ± 12.8 & |
| IVRT | 15.7± 1.4 | 23.5 ± 1.7 * | 19.6 ± 1.8 & |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-H.; Lin, C.-J.; Hang, C.-L.; Chen, W.-Y. Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice. Cells 2019, 8, 865. https://doi.org/10.3390/cells8080865
Tsai T-H, Lin C-J, Hang C-L, Chen W-Y. Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice. Cells. 2019; 8(8):865. https://doi.org/10.3390/cells8080865
Chicago/Turabian StyleTsai, Tzu-Hsien, Cheng-Jei Lin, Chi-Ling Hang, and Wei-Yu Chen. 2019. "Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice" Cells 8, no. 8: 865. https://doi.org/10.3390/cells8080865