The Latin American DILI Registry Experience: A Successful Ongoing Collaborative Strategic Initiative
Abstract
:1. Introduction
2. What Were Our Goals?
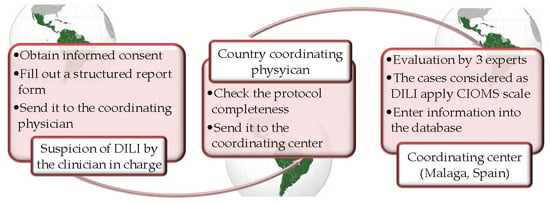
3. How Was the Project Implemented, and What Have We Attained so Far?
4. Preliminary Results from Our Registry
5. Where Do We Stand Today and What Are We Planning for the Future?
6. Summary and Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| DILI | Drug induced liver injury |
| LA | Latin America |
| ALEH | Latin American Association for Liver Study |
| CIOMS | Council for International Organizations of Medical Scientists |
| RUCAM | Roussel Uclaf Causality Assessment Method |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| GWA | genome wide association |
| HDS | herbals and dietary supplements |
References
- Andrade, R.J.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10 year period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, H.; Murata, Y.; Horiike, N.; Fukui, H.; Onji, M. Drug-induced liver injury in Japan: An analysis of 1676 cases between 1997 and 2006. Hepatol. Res. 2009, 39, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterology 2015, 148, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.; Bessone, F.; Sanchez, A.; di Pace, M.; Brahm, J.; Zapata, R.A.; Chirino, R.; Dávalos, M.; Méndez-Sánchez, N.; Arrese, M.; et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann. Hepatol. 2014, 13, 231–238. [Google Scholar] [PubMed]
- Leyva-Flores, R.; Erviti-Erice, J.; Kageyama-Escobar, M.L.; Arredondo, A. Medical prescription, drug Access and drug expenditure among health service users in Mexico. Salud Publ. Mex 1998, 40, 24–31. [Google Scholar]
- Bénard-Laribière, A.; Miremont-Salamé, G.; Pérault-Pochat, M.C.; Noize, P.; Haramburu, F. Incidence of hospital admissions due to adverse drug reactions in France: The EMIR study. Fundam. Clin. Pharmacol. 2015, 29, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Bégaud, B.; Martin, K.; Haramburu, F.; Moore, N. Rates of spontaneous reporting of adverse drug reactions in France. JAMA 2002, 288, 1588. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.S.; Hoffnagle, J.H. Categorization of drug implicated in causing liver injury: Critical assessment based upon case reports. Hepatology 2016, 63, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Colombato, L.; Fassio, E.; Reggiardo, M.; Vorobioff, J.; Tanno, H. The spectrum of nimesulide induce hepatotoxicity. An overview. Antiinflamm. Antiallergy Agents Med. Chem. 2010, 9, 355–365. [Google Scholar] [CrossRef]
- Bessone, F.; Colombato, L.; Frider, B.; Tsariktsian, G.; Bruguera, M.; Cohen, H.; Tsariktsian, G.; Hernández, N.; Bruguera, M.; Gualano, G.; et al. Cyproterone induced-liver-injury includes autoimmune hepatitis and a spectrum of varying severity of acute hepatcellular damage. Basic Clin. Pharmacol. Toxicol. 2011, 109, 32–40. [Google Scholar]
- Suzuki, A.; Andrade, R.J.; Bjornsson, E.; Lucena, M.I.; Lee, W.M.; Yuen NA Hunt, C.M.; Freston, J.W. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in vigibase. Drug Saf. 2010, 33, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Zapata, R.; Sanhueza, E.; Gómez, F.; Contreras, J.; Uribe, M.; Humeres, R.; Martínez, A.; Rius, M.; Basaez, A.M.; Rios, H.; et al. Falla hepática fulminante en adultos. Etiología, pronóstico y evolución de pacientes evaluados en dos unidades de trasplante hepático en Chile durante una década. Gastroenterol. Latinoam. 2012, 23, S40. [Google Scholar]
- Medicines Control Agency, Department of Health. Consultation Letter MLX231. In Analgesic Medicines Available Without Prescription: Proposed Changes to Product Information and Sale or Supply of Paracetamol; Department of Health: London, UK, 1996. [Google Scholar]
- Hawkins, L.C.; Edwards, J.N.; Dargan, P.I. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom. Drug Saf. 2007, 30, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Limiting Acetaminophen’s Strength in Prescription Medicines. All manufacturers of prescription combination drug products with more than 325 mg of acetaminophen have discontinued marketing. Available online: http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm165107.htm (accessed on 20 September 2015).
- Teschke, R.; Wolff, W.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: A tabular compilation of reported cases. Liver Int. 2012, 32, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Long, H.; Schwarzenboeck, A.; Schmidt-Taenzer, W.; Genthner, A.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Traditional Chinese medicine and herbal hepatotoxicity: A tabular compilation of reported cases. Ann. Hepatol. 2015, 14, 7–19. [Google Scholar] [PubMed]
- Zhou, Y.; Yang, L.; Liao, Z.; He, X.; Zhou, Y.; Guo, H. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21,789 patients. Eur. J. Gastroenterol. Hepatol. 2013, 25, 825–829. [Google Scholar] [CrossRef] [PubMed]
- García-Cortés, M.; Borraz, Y.; Lucena, M.I.; Peláez, G.; Salmerón, J.; Diago, M.; Martínez-Sierra, M.C.; Navarro, J.M.; Planas, R.; Soria, M.J.; et al. Liver injury induced by “natural remedies”: An analysis of cases submitted to the Spanish Liver Toxicity Registry. Rev. Esp. Enferm. Dig. 2008, 11, 688–695. [Google Scholar]
- Lucena, M.I.; Cohen, H.; Hernandez, N.; Bessone, F.; Dacoll, C.; Stephens, C.; Borraz, Y.; Ulzurrun, E.; Bruguera, M.; Andrade, J.R. Hepatotoxicidad, un problema real con especificidades locales: Hacia la creación de una Red Hispano Latinoamericana de Hepatotoxicidad. Gastroenterol. Hepatol. 2011, 34, 361–368. [Google Scholar] [CrossRef]
- Bessone, F.; Hernandez, N.; Davalos, M.; Parana, R.; Schinoni, M.; Lizarzabal, M.; Kershenobich, D.; Loaeza, A.; Arrese, M.; Chirino, R.A.; et al. Building a spanish Latin American network on drug induced liver injury: Much to get from a joint collaborative initiative. Ann. Hepatol. 2012, 11, 544–549. [Google Scholar] [PubMed]
- Asociación Latinoamericana para el estudio del Hígado. Available online: http://www.alehlatam.org (accessed on 20 September 2015).
- Jacobsen, K.H.; Wiersma, S.T. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccination 2010, 28, 6653–6657. [Google Scholar] [CrossRef] [PubMed]
- Echevarría, J.M.; González, J.E.; Lewis-Ximenez, L.L.; Dos Santos, D.R.; Munné, M.S.; Pinto, M.A.; Pujol, F.H.; Rodríguez-Lay, L.A. Hepatitis E virus infection in Latin America: A review. J. Med. Virol. 2013, 85, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Cruells, M.R.; Mescia, G.; Gaibisso, R.; Ramírez, M.; Gutiérrez, M.; Kohen, S.; González, M.; Russi, J.; Chiparelli, H.; Ucar, L.; et al. Epidemiological study of hepatitis A and E viruses in different populations in Uruguay. Gastroenterol. Hepatol. 1997, 20, 295–298. [Google Scholar] [PubMed]
- Mendizabal, M.; Marciano, S.; Videla, M.G.; Anders, M.; Zerega, A.; Balderramo, D.C.; Chan, D.; Barrabino, M.; Gil, O.; Mastai, R.; et al. Changing etiologies and outcomes of acute liver failure: Perspectives from 6 transplant centers in Argentina. Liver Transpl. 2014, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Bessone, F.; Lucena, M.I.; Roma, M.; Stephens, C.; Medina-Cáliz, I.; Frider, B.; Tsariktsian, G.; Hernández, N.; Bruguera, M.; Gualano, G.; et al. Cyproterone acetate induces a wide spectrum of liver damage including corticosteroid-responsive hepatitis: Report of 22 cases. Liver Int. 2016, 36, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Robles-Diaz, M.; Gonzalez-Jimenez, A.; Medina-Caliz, I.; Stephens, C.; García-Cortes, M.; García-Muñoz, B.; Ortega-Alonso, A.; Blanco-Reina, E.; Gonzalez-Grande, R.; Jimenez-Perez, M.; et al. Distinct phenotype of Hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment. Pharmacol. Ther. 2015, 41, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Robles-Diaz, M.; Lucena, M.I.; Kaplowitz, N.; Stephens, C.; Medina-Cáliz, I.; González-Jimenez, A.; Ulzurrun, E.; Gonzalez, A.F.; Fernandez, M.C.; Romero-Gómez, M.; et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014, 147, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Hernández, N.; Sánchez, A.; di Pace, M.; Gualano, G.; Arrese, M.; Ruiz, A.; del Castillo, A.L.; Girala, M.A.; Carrera, E.; et al. A comparative analysis of the Spanish and Latin-American prospective drug-induced liver injury (DILI) networks. Hepatology 2015, 62, 504A. [Google Scholar]
- Lucena, M.I.; Molokhia, M.; Shen, Y.; Urban, T.J.; Aithal, G.P.; Andrade, R.J.; Day, C.P.; Ruiz-Cabello, F.; Donaldson, P.T.; Stephens, C.; et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 2011, 141, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Donaldson, P.T.; Bhatnagar, P.; Shen, Y.; Pe’er, I.; Floratos, A.; Daly, M.J.; Goldstein, D.B.; John, S.; Nelson, M.R.; et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009, 41, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.B.; Lewitzky, S.; Leroy, E.; Yang, F.; Zhao, X.; Klickstein, L.; Wright, T.M.; Meyer, J.; Paulding, C.A. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat. Genet. 2010, 42, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Spraggs, C.F.; Budde, L.R.; Briley, L.P.; Bing, N.; Cox, C.J.; King, K.S.; Whittaker, J.C.; Mooser, V.E.; Preston, A.J.; Stein, S.H.; et al. HLA-DQA1*02:01 is a major risk factor for lapatanib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011, 29, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Stephens, C.; Robles-Diaz, M.; Medina-Caliz, I.; Ortega-Alonso, A.; Lucena, M.I.; Aithal, G.P.; Andrade, R.J. Pro-Euro-DILI Registry: A collaborative effort to enhance the understanding of DILI. J. Hepatol. submitted for publication. 2016. [Google Scholar]
- Aithal, G.P.; Grove, J.; Bjornsson, E.; Lucena, M.I.; Andrade, R.J.; Hallberg, P.; Stephens, C.; Par, H.; Maitland-van der Zee, A.H.; Martin, J.H.; et al. HLA-A*33:01 is strongly associated with drug-induced liver injury (DILI) due to terbinafine and several other unrelated compounds. Hepatology 2015, 62, 325A–326A. [Google Scholar]

| Therapeutic Class | n (%) | ALF/OLT n (%) | Individual Agent |
|---|---|---|---|
| NSAIDs and anti-rheumatic drugs | 62 (32) | 16 (26) | Nimesulide, Piroxicam, Diclofenac, Naproxen |
| Anti-infectious | 37 (19) | 11 (30) | Nitrofurantoin, Isoniazid, Trovofloxacin, Clarithromycin |
| Genito-urinary system and sex hormones | 34 (18) | 14 (22) | Progesterone, Cyproterone Acetate, Flutamide |
| Antineoplastic and immunomodulators | 10 (5) | 2 (3) | Imatinib, Tamoxifen |
| Anticonvulsants | 7 (4) | 3 | Valproic Acid, Phenytoin |
| Cardiovascular system | 6 (3) | 1 | Methyldopa |
| Anti-virals | 5 (3) | 1 | Nevirapine |
| Anesthetics | 5 (3) | 5 | Halothane |
| Antithyroid | 5 (3) | 4 | Propylthiouracil |
| Other groups | 20 | 5 | Ketoconazole, Griseofulvin, Disulfiram, Mycophenolate |
| Individual Agent (n) | Latin DILI Network (LATINDILIN) Registry (n 206) | Spanish DILI Registry (n 867) * | DILIN Study (n 899) |
|---|---|---|---|
| Amoxicillin-clavulanate | 20 | 186 | 91 |
| Diclofenac | 13 | 16 | 12 |
| Nimesulide | 11 | 9 | - |
| Nitrofurantoin | 11 | - | 42 |
| Cyproterone acetate | 9 | 3 | - |
| Ibuprofen | 7 | 22 | 1 |
| RIP + INH + PIZ# | 7 | 29 | - |
| Carbamazepine | 5 | 8 | 4 |
| Phenytoin | 4 | 3 | 12 |
| Thiamazole | 4 | 7 | 3 |
| Variables | LATINDILI Registry (200) | Spanish DILI Registry (867) | p Value |
|---|---|---|---|
| Mean age (years) | 51 | 54 | 0.02 |
| Female (%) | 59 | 49 | 0.01 |
| Jaundice (%) | 67 | 68 | NS |
| Fatal cases (%) | 4.6 | 4 | NS |
| Hepatocellular damage (%) | 54 | 63 | 0.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessone, F.; Hernandez, N.; Lucena, M.I.; Andrade, R.J.; On behalf of the Latin DILI Network (LATINDILIN) and Spanish DILI Registry. The Latin American DILI Registry Experience: A Successful Ongoing Collaborative Strategic Initiative. Int. J. Mol. Sci. 2016, 17, 313. https://doi.org/10.3390/ijms17030313
Bessone F, Hernandez N, Lucena MI, Andrade RJ, On behalf of the Latin DILI Network (LATINDILIN) and Spanish DILI Registry. The Latin American DILI Registry Experience: A Successful Ongoing Collaborative Strategic Initiative. International Journal of Molecular Sciences. 2016; 17(3):313. https://doi.org/10.3390/ijms17030313
Chicago/Turabian StyleBessone, Fernando, Nelia Hernandez, M. Isabel Lucena, Raúl J. Andrade, and On behalf of the Latin DILI Network (LATINDILIN) and Spanish DILI Registry. 2016. "The Latin American DILI Registry Experience: A Successful Ongoing Collaborative Strategic Initiative" International Journal of Molecular Sciences 17, no. 3: 313. https://doi.org/10.3390/ijms17030313