Spinal Cord T-Cell Infiltration in the Rat Spared Nerve Injury Model: A Time Course Study
Abstract
:1. Introduction
2. Results
2.1. SNI Upregulates Genes Related to Microglial Reactivity
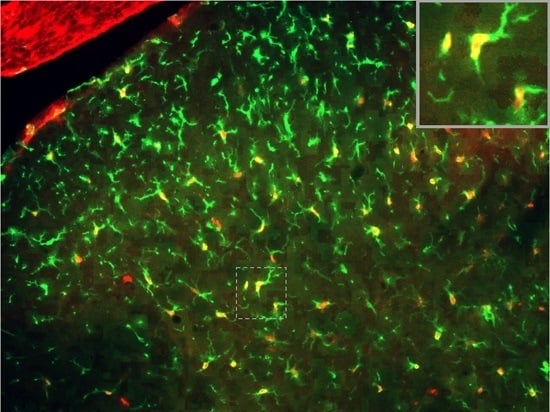
2.2. SNI Induces Microglial Activation and Proliferation
2.3. Inflammatory Genes Are Regulated after Peripheral Nerve Injury
2.4. T-Cells Do Not Infiltrate the Spinal Cord after SNI
2.5. T-Cells Infiltrate the Spinal Cord after SCI
3. Discussion
4. Materials and Methods
4.1. Animal Surgery
4.2. Immunohistochemistry
4.3. RT-qPCR and RT-qPCR Array
4.4. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BrdU | Bromodeoxyuridine |
| CI | Confidence interval |
| CCI | Chronic constriction injury |
| CHUV | Centre Hospitalier Universitaire Vaudois |
| CNS | Central nervous system |
| LPS | Lipopolysaccharides |
| MAC | Membrane attack complex |
| MDPI | Multidisciplinary Digital Publishing Institute |
| mRNA | Messenger ribonucleic acid |
| PBS | Phosphate buffer saline |
| PSNL | Partial sciatic nerve ligation |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SNI | Spared nerve injury |
| SCI | Spinal cord injury |
| TLR | Toll-like receptor |
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Russa, F.L.; Bennett, D.L. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci. 2015, 16, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Salter, M.W. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav. Immun. 2007, 21, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: Different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain 2009, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, X.Q.; Echeverry, S.; Mogil, J.S.; de Koninck, Y.; Rivest, S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 2007, 27, 45. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.M.; Hickey, W.F.; Rutkowski, M.D.; Pahl, J.L.; DeLeo, J.A. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: Potential relationship to neuropathic pain. Pain 2002, 100, 163–170. [Google Scholar] [CrossRef]
- Hu, P.; Bembrick, A.L.; Keay, K.A.; McLachlan, E.M. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav. Immun. 2007, 21, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; DeLeo, J.A. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008, 38, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Moss, A.; Latremoliere, A.; Johnston, C.; Verma-Gandhu, M.; Herbert, T.A.; Barrett, L.; Brenner, G.J.; Vardeh, D.; Woolf, C.J.; et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 2009, 29, 14415–14422. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.F.; Moalem-Taylor, G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 2011, 1405, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Kim, C.F.; Perera, C.J.; Moalem-Taylor, G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 2012, 153, 1916–1931. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G. The new statistics: Why and how. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; de Palo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.S.; Costigan, M.; Brenner, G.J.; Ma, C.H.; Scholz, J.; Moss, A.; Allchorne, A.J.; Stahl, G.L.; Woolf, C.J. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J. Neurosci. 2007, 27, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Figshare. Available online: http://figshare.com/s/e1a0e64486fd11e5b72906ec4b8d1f61 (accessed on 5 November 2015).
- Jia, G.Q.; Gonzalo, J.A.; Lloyd, C.; Kremer, L.; Lu, L.; Martinez, A.C.; Wershil, B.K.; Gutierrez-Ramos, J.C. Distinct expression and function of the novel mouse chemokine monocyte chemotactic protein-5 in lung allergic inflammation. J. Exp. Med. 1996, 184, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.B.; Cerovic, V.; Milling, S.W.; Graham, G.J.; Hansell, C.A.; Nibbs, R.J. Characterization of conventional and atypical receptors for the chemokine CCL2 on mouse leukocytes. J. Immunol. 2014, 193, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Carter, S.; Hofer, M.J.; Campbell, I.L. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—A tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010, 36, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Chen, C.L.; Lin, Y.S.; Yeh, T.M.; Tsai, T.T.; Hong, M.Y.; Lin, C.F. Macrophage migration inhibitory factor triggers chemotaxis of CD74+CXCR2+ NKT cells in chemically induced IFN-γ-mediated skin inflammation. J. Immunol. 2014, 193, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chhor, V.; Le Charpentier, T.; Lebon, S.; Ore, M.V.; Celador, I.L.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Savman, K.; et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 2013, 32, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Lue, L.F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Malcangio, M. Microglial signalling mechanisms: Cathepsin S and Fractalkine. Exp. Neurol. 2012, 234, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Collier, J.G.M.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 2011, 145, 584–595. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, R.; Berta, T.; Old, E.; Ji, R.R.; Fitzgerald, M. Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation. J. Neurosci. 2015, 35, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Penzkover, K.R.; Soderquist, R.G.; Mahoney, M.J. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Svensson, C.I. Role of extracellular damage-associated molecular pattern molecules (DAMPs) as mediators of persistent pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 251–279. [Google Scholar] [PubMed]
- Yu, L.; Wang, L.; Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 243, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, M.A.; Cho, I.H.; Kim, M.S.; Lee, S.; Jo, E.K.; Choi, S.Y.; Park, K.; Kim, J.S.; Akira, S.; et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, K.; Derkow, K.; Dembny, P.; Kruger, C.; Schott, E.; Lehnardt, S. The impact of single and pairwise Toll-like receptor activation on neuroinflammation and neurodegeneration. J. Neuroinflamm. 2014, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; Yang, M.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Holscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Nishi, R.A.; Liu, H.; Chu, Y.; Hamamura, M.; Su, M.Y.; Nalcioglu, O.; Anderson, A.J. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma 2007, 24, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]






| Antibody | Source | Target | Concentration |
|---|---|---|---|
| Mouse anti-CD2 | Serotec, UK | T-cells | 1:250 |
| Mouse anti-CD8 | Abcam, USA | CD8+ T-cells | 1:250 |
| Rabbit anti-Iba1 | Wako, USA | Activated microglia | 1:2000 |
| Mouse anti-GFAP | Millipore, USA | Activated astrocytes | 1:1500 |
| Rat anti-BrdU | Abcam, USA | Proliferating cells | 1:500 |
| Antibody | Source | Concentration |
|---|---|---|
| Alexa 488-labelled goat anti-mouse | Molecular Probes, UK | 1:500 |
| Alexa 488-labelled donkey anti-rabbit | Molecular Probes, UK | 1:500 |
| Cy3-labelled donkey anti-mouse | Jackson, USA | 1:500 |
| Cy3-labelled donkey anti-rat | Jackson, USA | 1:500 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gattlen, C.; Clarke, C.B.; Piller, N.; Kirschmann, G.; Pertin, M.; Decosterd, I.; Gosselin, R.-D.; Suter, M.R. Spinal Cord T-Cell Infiltration in the Rat Spared Nerve Injury Model: A Time Course Study. Int. J. Mol. Sci. 2016, 17, 352. https://doi.org/10.3390/ijms17030352
Gattlen C, Clarke CB, Piller N, Kirschmann G, Pertin M, Decosterd I, Gosselin R-D, Suter MR. Spinal Cord T-Cell Infiltration in the Rat Spared Nerve Injury Model: A Time Course Study. International Journal of Molecular Sciences. 2016; 17(3):352. https://doi.org/10.3390/ijms17030352
Chicago/Turabian StyleGattlen, Christophe, Christine B. Clarke, Nicolas Piller, Guylène Kirschmann, Marie Pertin, Isabelle Decosterd, Romain-Daniel Gosselin, and Marc R. Suter. 2016. "Spinal Cord T-Cell Infiltration in the Rat Spared Nerve Injury Model: A Time Course Study" International Journal of Molecular Sciences 17, no. 3: 352. https://doi.org/10.3390/ijms17030352