Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway
Abstract
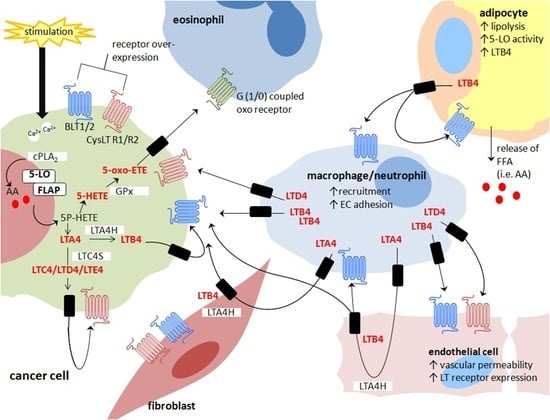
:1. Introduction
2. 5-Lipoxygenase Pathway in Cancer
3. Blockade of Leukotriene Synthesis in Cancer Models
4. 5-Lipoxygenase and Immune Regulation
4.1. 5-Lipoxygenase Signalling and Infiltrating Myeloid Derived Cells
4.2. 5-Lipoxygenase Signalling and Lymphocytes
5. 5-Lipoxygenase and Tumour Angiogenesis
5.1. Cancer Cell Derived 5-Lipoxygenase in Angiogenesis
5.2. 5-Lipoxygenase Signalling and VEGF-Mediated Angiogenesis
5.3. Endothelial Cell Transcellular Synthesis of Leukotrienes
5.4. 5-Lipoxygenase and Cyclooxygenase-2 Shunting in Tumour Angiogenesis
6. 5-Lipoxygenase and Adipose Tissue
7. 5-Lipoxygenase and Cancer-Associated Fibroblasts
8. Conclusions
Conflicts of Interest
Abbreviations
| 12-LO | 12-Lipoxygenase |
| 15-LO | 15-Lipoxygenase |
| 5-LO | 5-Lipoxygenase |
| AA | Arachidonic acid |
| BAL | Bronchial alveolar lavages |
| COX | Cyclooxygenase |
| ECM | Extracellular matrix |
| FFA | Free fatty acids |
| FLAP | 5-Lipoxygenase associated protein |
| HETE | Hydroxyeicosatetraenoic acid |
| LT | Leukotriene |
| MDSC | Myeloid-derived suppressor cells |
| PMNL | Polymorphonuclear leukocytes |
| TAM | Tumour associated macrophages |
| TME | Tumour microenvironment |
References
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Spagnoli, V.; Tardif, J.C.; L’Allier, P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015, 240, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.B.; Pratico, D. The 5-lipoxygenase pathway: Oxidative and inflammatory contributions to the Alzheimer’s disease phenotype. Front. Cell Neurosci. 2014, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Tersey, S.A.; Bolanis, E.; Holman, T.R.; Maloney, D.J.; Nadler, J.L.; Mirmira, R.G. Minireview: 12-Lipoxygenase and Islet beta-Cell Dysfunction in Diabetes. Mol. Endocrinol. 2015, 29, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Knab, L.M.; Grippo, P.J.; Bentrem, D.J. Involvement of eicosanoids in the pathogenesis of pancreatic cancer: The roles of cyclooxygenase-2 and 5-lipoxygenase. World J. Gastroenterol. 2014, 20, 10729–10739. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Walther, M.; Kuban, R.J. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002, 68–69, 263–290. [Google Scholar] [CrossRef]
- Klil-Drori, A.J.; Ariel, A. 15-Lipoxygenases in cancer: A double-edged sword? Prostaglandins Other Lipid Mediat. 2013, 106, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kelavkar, U.P.; Cohen, C. 15-lipoxygenase-1 expression upregulates and activates insulin-like growth factor-1 receptor in prostate cancer cells. Neoplasia 2004, 6, 41–52. [Google Scholar] [CrossRef]
- Kelavkar, U.P.; Nixon, J.B.; Cohen, C.; Dillehay, D.; Eling, T.E.; Badr, K.F. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis 2001, 22, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Middleton, M.K.; Zukas, A.M.; Rubinstein, T.; Jacob, M.; Zhu, P.; Zhao, L.; Blair, I.; Pure, E. Identification of 12/15-lipoxygenase as a suppressor of myeloproliferative disease. J. Exp. Med. 2006, 203, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Nixon, J.B.; Kim, K.S.; Lamb, P.W.; Bottone, F.G.; Eling, T.E. 15-Lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shureiqi, I.; Wojno, K.J.; Poore, J.A.; Reddy, R.G.; Moussalli, M.J.; Spindler, S.A.; Greenson, J.K.; Normolle, D.; Hasan, A.A.; Lawrence, T.S.; et al. Decreased 13-S-hydroxyoctadecadienoic acid levels and 15-lipoxygenase-1 expression in human colon cancers. Carcinogenesis 1999, 20, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Shureiqi, I.; Wu, Y.; Chen, D.; Yang, X.L.; Guan, B.; Morris, J.S.; Yang, P.; Newman, R.A.; Broaddus, R.; Hamilton, S.R.; et al. The critical role of 15-lipoxygenase-1 in colorectal epithelial cell terminal differentiation and tumorigenesis. Cancer Res. 2005, 65, 11486–11492. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Esworthy, R.; Bai, W.; Gu, J.L.; Wilczynski, S.; Nadler, J. Increased 12-lipoxygenase expression in breast cancer tissues and cells. Regulation by epidermal growth factor. J. Clin. Endocrinol. Metab. 1997, 82, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Watkins, G.; Douglas-Jones, A.; Mansel, R.E. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Suraneni, M.V.; Moore, J.R.; Zhang, D.; Badeaux, M.; Macaluso, M.D.; DiGiovanni, J.; Kusewitt, D.; Tang, D.G. Tumor-suppressive functions of 15-Lipoxygenase-2 and RB1CC1 in prostate cancer. Cell Cycle 2014, 13, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nie, D. Tumor-suppressing 15-lipoxygenase-2: Time for prime time? Cell Cycle 2014, 13, 1836–1837. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Grignon, D.J.; Chbihi, T.; Zacharek, A.; Chen, Y.Q.; Sakr, W.; Porter, A.T.; Crissman, J.D.; Pontes, J.E.; Powell, I.J.; et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology 1995, 46, 227–237. [Google Scholar] [CrossRef]
- Nithipatikom, K.; Isbell, M.A.; See, W.A.; Campbell, W.B. Elevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseases. Cancer Lett. 2006, 233, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Douglas-Jones, A.; Mansel, R.E. Levels of expression of lipoxygenases and cyclooxygenase-2 in human breast cancer. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 275–281. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Abdel, H.A.; Abdel, W.; Ahmed, A.M.; Wael, T.; Eiman, G. Expression of cyclooxygenase-2 and 12-lipoxygenase in human breast cancer and their relationship with HER-2/neu and hormonal receptors: Impact on prognosis and therapy. Indian J. Cancer 2006, 43, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Yoshimura, R.; Mitsuhashi, M.; Hase, T.; Tsuchida, K.; Takemoto, Y.; Kawahito, Y.; Sano, H.; Nakatani, T. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int. J. Oncol. 2004, 24, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Hayama, T.; Funao, K.; Kawahito, Y.; Sano, H.; Takemoto, Y.; Nakatani, T.; Yoshimura, R. Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate cancer cell growth through apoptosis. Oncol. Rep. 2007, 18, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Douglas-Jones, A.G.; Mansel, R.E. Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP) has prognostic and survival significance in patients with breast cancer. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Ding, X.Z.; Tong, W.G.; Schneider, M.B.; Standop, J.; Friess, H.; Buchler, M.W.; Pour, P.M.; Adrian, T.E. 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am. J. Pathol. 2002, 161, 421–428. [Google Scholar] [CrossRef]
- Barresi, V.; Grosso, M.; Vitarelli, E.; Tuccari, G.; Barresi, G. 5-Lipoxygenase is coexpressed with Cox-2 in sporadic colorectal cancer: A correlation with advanced stage. Dis. Colon Rectum 2007, 50, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Iversen, P.; Cluck, M.W.; Knezetic, J.A.; Adrian, T.E. Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 1999, 261, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, H.; Li, M.; Li, B.; Gao, W.; Shao, Q.; Fan, B.; Zhao, F.; Wang, Q.; Xie, Q.; et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene 2014, 34, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B-cells promote breast cancer metastasis by converting resting CD4+ T-cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef] [PubMed]
- Wejksza, K.; Lee-Chang, C.; Bodogai, M.; Bonzo, J.; Gonzalez, F.J.; Lehrmann, E.; Becker, K.; Biragyn, A. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B-cells via peroxisome proliferator-activated receptor alpha. J. Immunol. 2013, 190, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Boger, P.C.; Shutt, J.D.; Neale, J.R.; Wilson, S.J.; Bateman, A.C.; Holloway, J.W.; Patel, P.; Sampson, A.P. Increased expression of the 5-lipoxygenase pathway and its cellular localization in Barrett’s adenocarcinoma. Histopathology 2012, 61, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125 Pt 23, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Neels, J.G. A role for 5-lipoxygenase products in obesity-associated inflammation and insulin resistance. Adipocyte 2013, 2, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Fischer, A.S.; Metzner, J.; Steinbrink, S.D.; Roos, J.; Ruthardt, M.; Maier, T.J. 5-Lipoxygenase: Underappreciated role of a pro-inflammatory enzyme in tumorigenesis. Front. Pharmacol. 2010, 1, 143. [Google Scholar] [CrossRef] [PubMed]
- Kubavat, A.H.; Khippal, N.; Tak, S.; Rijhwani, P.; Bhargava, S.; Patel, T.; Shah, N.; Kshatriya, R.R.; Mittal, R. A randomized, comparative, multicentric clinical trial to assess the efficacy and safety of zileuton extended-release tablets with montelukast sodium tablets in patients suffering from chronic persistent asthma. Am. J. Ther. 2013, 20, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.R.; Carter, G.W.; Bell, R.L.; Moore, J.L.; Brooks, D.W. Clinical activity of leukotriene inhibitors. Int. J. Immunopharmacol. 1995, 17, 147–156. [Google Scholar] [CrossRef]
- Knorr, B.; Matz, J.; Bernstein, J.A.; Nguyen, H.; Seidenberg, B.C.; Reiss, T.F.; Becker, A. Montelukast for chronic asthma in 6- to 14-year-old children: A randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA 1998, 279, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Dennis, R.; Ernst, P.; Sheehy, O.; Wood-Dauphinee, S. Effectiveness of the leukotriene receptor antagonist zafirlukast for mild-to-moderate asthma. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1997, 126, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.W.; Coffey, M.J.; Brock, T.G.; Singer, I.I.; Peters-Golden, M. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J. Clin. Investig. 1995, 95, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Radmark, O.; Samuelsson, B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem. Biophys. Res. Commun. 2010, 396, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Klemm, J.; Samuelsson, B.; Radmark, O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. USA 2000, 97, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Flamand, N.; Ming, L.; Peters-Golden, M.; Brock, T.G. Phsophorylation of serine 271 on 5-lipoxygenase and its role in nuclear export. J. Biol. Chem. 2009, 284, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.A.; Abramovitz, M.; Cox, M.E.; Wong, E.; Charleson, S.; Perrier, H.; Wang, Z.; Prasit, P.; Vickers, P.J. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993, 318, 277–281. [Google Scholar] [CrossRef]
- Abramovitz, M.; Wong, E.; Cox, M.E.; Richardson, C.D.; Li, C.; Vickers, P.J. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur. J. Biochem. 1993, 215, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Funk, C.D. Enzymes involved in the biosynthesis of leukotriene B4. J. Biol. Chem. 1989, 264, 19469–19472. [Google Scholar] [PubMed]
- Schwenk, U.; Schroder, J.M. 5-Oxo-eicosanoids are potent eosinophil chemotactic factors. Functional characterization and structural requirements. J. Biol. Chem. 1995, 270, 15029–15036. [Google Scholar] [CrossRef] [PubMed]
- Rokach, J.; Powell, W.S. Biochemistry, biology and chemistry of the 5-lipoxygenase product 5-oxo-ETE. Prog. Lipid Res. 2005, 44, 154–183. [Google Scholar]
- Penrose, J.F.; Gagnon, L.; Goppelt-Struebe, M.; Myers, P.; Lam, B.K.; Jack, R.M.; Austen, K.F.; Soberman, R.J. Purification of human leukotriene C4 synthase. Proc. Natl. Acad. Sci. USA 1992, 89, 11603–11606. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Gleason, M.M.; Togias, A. Cysteinyl leukotrienes: Multi-functional mediators in allergic rhinitis. Clin. Exp. Allergy 2006, 36, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [PubMed]
- Lehmann, C.; Homann, J.; Ball, A.K.; Blöcher, R.; Kleinschmidt, T.K.; Basavarajappa, D.; Angioni, C.; Ferreirós, N.; Häfner, A.K.; Rådmark, O.; et al. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 2015, 29, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- Shutt, J.D.; Boger, P.; Neale, J.R.; Patel, P.; Sampson, A.P. Activity of the leukotriene pathway in Barrett’s metaplasia and oesophageal adenocarcinoma. Inflamm. Res. 2012, 61, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Sakamoto, K.; Bostwick, D.G.; Mukhtar, H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer 2001, 91, 737–743. [Google Scholar] [CrossRef]
- Wasilewicz, M.P.; Kolodziej, B.; Bojulko, T.; Kaczmarczyk, M.; Sulzyc-Bielicka, V.; Bielicki, D.; Ciepiela, K. Overexpression of 5-lipoxygenase in sporadic colonic adenomas and a possible new aspect of colon carcinogenesis. Int. J. Colorectal. Dis. 2010, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Grippo, P.; Ding, X.Z.; Rao, S.M.; Buchler, M.W.; Friess, H.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005, 65, 6011–6016. [Google Scholar] [CrossRef] [PubMed]
- Tager, A.M.; Luster, A.D. BLT1 and BLT2: The leukotriene B(4) receptors. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 123–134. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. J. Immunol. 2004, 173, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Russano, A.M.; Piattoni, S.; Agea, E.; Bistoni, O.; de Benedictis, D.; de Benedictis, F.M. Biological effects of montelukast, a cysteinyl-leukotriene receptor-antagonist, on T lymphocytes. Clin. Exp. Allergy 2004, 34, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.M.; Cho, K.J.; Kim, E.Y.; Choi, M.H.; Chung, B.C.; Kim, J.H. Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp. Mol. Med. 2011, 43, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Mezhybovska, M.; Lorinc, E.; Fernebro, E.; Nilbert, M.; Sjolander, A. Low expression of CysLT1R and high expression of CysLT2R mediate good prognosis in colorectal cancer. Eur. J. Cancer 2010, 46, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Liu, J.; Ehrnstrom, R.; Manjer, J.; Jirstrom, K.; Andersson, T.; Sjolander, A. Cysteinyl leukotriene receptor expression pattern affects migration of breast cancer cells and survival of breast cancer patients. Int. J. Cancer 2011, 129, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Venerito, M.; Kuester, D.; Harms, C.; Schubert, D.; Wex, T.; Malfertheiner, P. Upregulation of Leukotriene Receptors in Gastric Cancer. Cancers 2011, 3, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Osman, T.; Esposito, I.; Giese, N.; Rao, S.M.; Ding, X.Z.; Tong, W.G.; Buchler, M.W.; Yokomizo, T.; Friess, H.; et al. BLT2 is expressed in PanINs, IPMNs, pancreatic cancer and stimulates tumour cell proliferation. Br. J. Cancer 2008, 99, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Lee, J.W.; Cho, S.H.; Seo, J.M.; Kim, J.H. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler Thromb. Vasc. Biol. 2009, 29, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H.; Song, H.; Woo, C.H.; Kim, H.; Kim, J.H. Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene 2004, 23, 9259–9268. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Seo, J.M.; Cho, K.J.; Kim, J.H. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene 2010, 29, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Myers, C.E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Cao, R.; Yang, Z.; Liu, T.; Wang, Y.; Wang, X. Inhibitor of 5-lipoxygenase, zileuton, suppresses prostate cancer metastasis by upregulating E-cadherin and paxillin. Urology 2013, 82, 1452.e7–1452.e14. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, S.; Thamilselvan, V.; Brodie, C.; Ghosh, J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon. Biochim. Biophys. Acta 2011, 1813, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, S.; Chakraborty, D.; Chitale, D.; Sears, R.; Ghosh, J. Inhibition of 5-lipoxygenase selectively triggers disruption of c-myc signaling in prostate cancer cells. J. Biol. Chem. 2015, 290, 4994–5006. [Google Scholar] [CrossRef] [PubMed]
- Saraveswaran, S.; Varma, N.; Morisetty, S.; Ghosh, J. Inhibition of 5-lipoxygenase downregulates stemness and kills prostate cancer stem cells by triggering apoptosis via activation of c-Jun N-terminal kinase. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Yoshimura, R.; Mitsuhashi, M.; Tsuchida, K.; Takemoto, Y.; Kawahito, Y.; Sano, H.; Nakatani, T. 5-Lipoxygenase inhibitors attenuate growth of human renal cell carcinoma and induce apoptosis through arachidonic acid pathway. Oncol. Rep. 2005, 14, 73–79. [Google Scholar] [PubMed]
- Ding, X.Z.; Tong, W.G.; Adrian, T.E. Multiple signal pathways are involved in the mitogenic effect of 5(S)-HETE in human pancreatic cancer. Oncology 2003, 65, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.G.; Ding, X.Z.; Witt, R.C.; Adrian, T.E. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol. Cancer Ther. 2002, 1, 929–935. [Google Scholar] [PubMed]
- Fischer, A.S.; Metzner, J.; Steinbrink, S.D.; Ulrich, S.; Angioni, C.; Geisslinger, G.; Steinhilber, D.; Maier, T.J. 5-Lipoxygenase inhibitors induce potent anti-proliferative and cytotoxic effects in human tumour cells independently of suppression of 5-lipoxygenase activity. Br. J. Pharmacol. 2010, 161, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Oancea, C.; Heinssmann, M.; Khan, D.; Held, H.; Kahnt, A.S.; Capelo, R.; la Buscato, E.; Proschak, E.; Puccetti, E.; et al. 5-Lipoxygenase is a candidate target for therapeutic management of stem cell-like cells in acute myeloid leukemia. Cancer Res. 2014, 74, 5244–5255. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Zhang, H.; Peng, C.; Li, S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat. Genet. 2009, 41, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Lippman, S.M.; Wu, T.T.; Xu, Y.; Liang, Z.D.; Swisher, S.; Zhang, H.; Cao, L.; Ajani, J.A.; Xu, X.C. Increased 5-lipoxygenase expression and induction of apoptosis by its inhibitors in esophageal cancer: A potential target for prevention. Carcinogenesis 2005, 26, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Melstrom, L.G.; Bentrem, D.J.; Salabat, M.R.; Kennedy, T.J.; Ding, X.Z.; Strouch, M.; Rao, S.M.; Witt, R.C.; Ternent, C.A.; Talamonti, M.S.; et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin. Cancer Res. 2008, 14, 6525–6530. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.C.; Khazaie, K.; Khan, M.W.; Strouch, M.J.; Krantz, S.B.; Phillips, J.; Blatner, N.R.; Hix, L.M.; Zhang, M.; Dennis, K.L.; et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res. 2011, 71, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.G. Regulating leukotriene synthesis: The role of nuclear 5-lipoxygenase. J. Cell. Biochem. 2005, 96, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Tavakoli Tameh, A.; Parent, C.A. Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. PLoS Biol. 2016, 14, e1002336. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Henderson, W.R., Jr. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Profita, M.; Melis, M.; Bonanno, A.; Paterno, A.; Mody, C.H.; Spatafora, M.; Ferraro, M.; Siena, L.; Vignola, A.M.; et al. LTB4 is present in exudative pleural effusions and contributes actively to neutrophil recruitment in the inflamed pleural space. Clin. Exp. Immunol. 2004, 135, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.C.; Strouch, M.J.; Krantz, S.B.; Heiferman, M.J.; Bentrem, D.J. Genetic deletion of 5-lipoxygenase increases tumor-infiltrating macrophages in Apc(Delta468) mice. J. Gastrointest. Surg. 2012, 16, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Qualls, J.E.; Murray, P.J. Tumor macrophages protective and pathogenic roles in cancer development. Curr. Top. Dev. Biol. 2011, 94, 309–328. [Google Scholar] [PubMed]
- Xing, Z.; Zganiacz, A.; Santosuosso, M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-alpha and nitric oxide from macrophages via IFN-gamma induction. J. Leukoc. Biol. 2000, 68, 897–902. [Google Scholar] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Kitadai, Y.; Tanaka, S.; Yoshihara, M.; Yasui, W.; Mukaida, N.; Haruma, K.; Chayama, K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int. J. Oncol. 2003, 22, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.S.; Flavell, R.A. Shielding the double-edged sword: Negative regulation of the innate immune system. J. Leukoc. Biol. 2004, 75, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Musa, M. Immune Mechanism: A ‘Double-Edged Sword’. Malays J. Med. Sci. 2013, 20, 61–67. [Google Scholar] [PubMed]
- Kilfeather, S. 5-lipoxygenase inhibitors for the treatment of COPD. Chest 2002, 121 (Suppl. S5), 197S–200S. [Google Scholar] [CrossRef] [PubMed]
- Kowal-Bielecka, O.; Distler, O.; Kowal, K.; Siergiejko, Z.; Chwiecko, J.; Sulik, A.; Gay, R.E.; Lukaszyk, A.B.; Gay, S.; Sierakowski, S. Elevated levels of leukotriene B4 and leukotriene E4 in bronchoalveolar lavage fluid from patients with scleroderma lung disease. Arthritis Rheumatol. 2003, 48, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Amat, M.; Barcons, M.; Mancebo, J.; Mateo, J.; Oliver, A.; Mayoral, J.F.; Fontcuberta, J.; Vila, L. Evolution of leukotriene B4, peptide leukotrienes, and interleukin-8 plasma concentrations in patients at risk of acute respiratory distress syndrome and with acute respiratory distress syndrome: Mortality prognostic study. Crit. Care Med. 2000, 28, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Tsu, I.H.; Dubinett, S.M.; Adams, B.; Sarafian, T.; Baratelli, F.; Roth, M.D.; Serio, K.J. Modulation of pulmonary leukotriene B4 production by cyclooxygenase-2 inhibitors and lipopolysaccharide. Clin. Cancer Res. 2004, 10, 6872–6878. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Tashkin, D.P.; Tsu, I.H.; Serio, K.J. Differential modulation of leukotriene B4 synthesis and degradation in human bronchoalveolar lavage cells by lipopolysaccharide and tobacco smoke. Cancer Prev. Res. 2008, 1, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Ohd, J.F.; Nielsen, C.K.; Campbell, J.; Landberg, G.; Lofberg, H.; Sjolander, A. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology 2003, 124, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Sand-Dejmek, J.; Sjolander, A. The inflammatory mediator leukotriene D(4) induces subcellular beta-catenin translocation and migration of colon cancer cells. Exp. Cell Res. 2014, 321, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, E.; Erdman, S.E.; Restaino, C.; Gurish, M.F.; Friend, D.S.; Gounari, F.; Lee, D.M.; Zhang, G.; Glickman, J.N.; Shin, K.; et al. Mast cells are an essential hematopoietic component for polyp development. Proc. Natl. Acad. Sci. USA 2007, 104, 19977–19982. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, E.; Heiferman, M.J.; Heiferman, J.R.; Shrivastav, M.; Vitello, D.; Blatner, N.R.; Knab, L.M.; Phillips, J.D.; Cheon, E.C.; Grippo, P.J.; et al. Zileuton, 5-lipoxygenase inhibitor, acts as a chemopreventive agent in intestinal polyposis, by modulating polyp and systemic inflammation. PLoS ONE 2015, 10, e0121402. [Google Scholar] [CrossRef] [PubMed]
- Knab, L.M.; Schultz, M.; Principe, D.R.; Mascarinas, W.E.; Gounaris, E.; Munshi, H.G.; Grippo, P.J.; Bentrem, D.J. Ablation of 5-lipoxygenase mitigates pancreatic lesion development. J. Surg. Res. 2015, 194, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mangalath, U.; Aslam, S.A.; Abdul Khadar, A.H.; Francis, P.G.; Mikacha, M.S.; Kalathingal, J.H. Recent trends in prevention of oral cancer. J. Int. Soc. Prev. Community Dent. 2014, 4 (Suppl. S3), S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, K. Risk factors in oral and oropharyngeal squamous cell carcinoma: A population-based case-control study in southern Sweden. Swed. Dent. J. Suppl. 2005, 179, 1–66. [Google Scholar]
- Guo, Y.; Wang, X.; Zhang, X.; Sun, Z.; Chen, X. Ethanol promotes chemically induced oral cancer in mice through activation of the 5-lipoxygenase pathway of arachidonic acid metabolism. Cancer Prev. Res. 2011, 4, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Ichimiya, S.; Kikuchi, T.; Saito, Y.; Matsumiya, H.; Ara, S.; Koshiba, S.; Zhang, J.; Hatate, C.; Tonooka, A.; et al. Arachidonate 5-lipoxygenase establishes adaptive humoral immunity by controlling primary B-cells and their cognate T-cell help. Am. J. Pathol. 2011, 178, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T-cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Erdag, G.; Schaefer, J.T.; Smolkin, M.E.; Deacon, D.H.; Shea, S.M.; Dengel, L.T.; Patterson, J.W.; Slingluff, C.L., Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012, 72, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qian, H.; Liu, Y.; Duan, L.; Li, Y.; Shi, G. The roles of regulatory B-cells in cancer. J. Immunol. Res. 2014, 2014, 215471. [Google Scholar] [CrossRef] [PubMed]
- Guriec, N.; Le Jossic-Corcos, C.; Simon, B.; Ianotto, J.C.; Tempescul, A.; Dreano, Y.; Salaun, J.P.; Berthou, C.; Corcos, L. The arachidonic acid-LTB4-BLT2 pathway enhances human B-CLL aggressiveness. Biochim. Biophys. Acta 2014, 1842, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Cook-Moreau, J.M.; El-Makhour Hojeij, Y.; Barrière, G.; Rabinovitch-Chable Héè, C.; Faucher, K.S.; Sturtz, F.G.; Rigaud, M.A. Expression of 5-lipoxygenase (5-LOX) in T lymphocytes. Immunology 2007, 122, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Prinz, I.; Gregoire, C.; Mollenkopf, H.; Aguado, E.; Wang, Y.; Malissen, M.; Kaufmann, S.H.; Malissen, B. The type 1 cysteinyl leukotriene receptor triggers calcium influx and chemotaxis in mouse alpha beta- and gamma delta effector T-cells. J. Immunol. 2005, 175, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, N.; Takeda, K.; Miyahara, S.; Taube, C.; Joetham, A.; Koya, T.; Matsubara, S.; Dakhama, A.; Tager, A.M.; Luster, A.D.; et al. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T-cells and airway hyperresponsiveness. J. Immunol. 2005, 174, 4979–4984. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Chheda, Z.; Jala, V.R.; Haribabu, B. Expression of Leukotriene B(4) receptor-1 on CD8(+) T-cells is required for their migration into tumors to elicit effective antitumor immunity. J. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Poczobutt, J.M.; Nguyen, T.T.; Hanson, D.; Li, H.; Sippel, T.R.; Weiser-Evans, M.C.; Gijon, M.; Murphy, R.C.; Nemenoff, R.A. Deletion of 5-Lipoxygenase in the Tumor Microenvironment Promotes Lung Cancer Progression and Metastasis through Regulating T Cell Recruitment. J. Immunol. 2016, 196, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Blatner, N.R.; Mulcahy, M.F.; Dennis, K.L.; Scholtens, D.; Bentrem, D.J.; Phillips, J.D.; Ham, S.; Sandall, B.P.; Khan, M.W.; Mahvi, D.M.; et al. Expression of RORgammat marks a pathogenic regulatory T-cell subset in human colon cancer. Sci. Transl. Med. 2012, 4, 164ra159. [Google Scholar] [CrossRef] [PubMed]
- Benard, M.; Straat, K.; Omarsdottir, S.; Leghmari, K.; Bertrand, J.; Davrinche, C.; Duga-Neulat, I.; Soderberg-Naucler, C.; Rahbar, A.; Casper, C. Human cytomegalovirus infection induces leukotriene B4 and 5-lipoxygenase expression in human placentae and umbilical vein endothelial cells. Placenta 2014, 35, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Loscalzo, J.; Zhang, Y.Y. 5-Lipoxygenase and human pulmonary artery endothelial cell proliferation. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H585–H593. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.; Tuder, R.M.; Wang, J.; Cool, C.D.; Lepley, R.A.; Voelkel, N.F. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1998, 157, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Walia, N.; Chandran, K.; Patel, K.; Veettil, M.V.; Marginean, A. The Kaposi’s sarcoma-associated herpesvirus (KSHV)-induced 5-lipoxygenase-leukotriene B4 cascade plays key roles in KSHV latency, monocyte recruitment, and lipogenesis. J. Virol. 2014, 88, 2131–2156. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012, 8, e1002866. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Johansson, A.S.; Sjostrom, M.; Wan, M.; Schroder, O.; Palmblad, J.; Haeggstrom, J.Z. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc. Natl. Acad. Sci. USA 2006, 103, 6913–6918. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.S.; Haeggstrom, J.Z.; Palmblad, J. Commonly used leukotriene B4 receptor antagonists possess intrinsic activity as agonists in human endothelial cells: Effects on calcium transients, adhesive events and mediator release. Prostaglandins Leukot. Essent. Fatty Acids 2011, 84, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Pardridge, W.M.; Vinters, H.V.; Black, K.L. Differential expression of arachidonate 5-lipoxygenase transcripts in human brain tumors: Evidence for the expression of a multitranscript family. Proc. Natl. Acad. Sci. USA 1992, 89, 9044–9048. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.W.; Jiang, X.F.; Chen, J.H.; Bai, J.S.; Dai, C.; Wang, Q.L.; Lu, J.Y.; Zhao, W.; Xin, R.; Liu, M.Y.; et al. Increased angiogenic capabilities of endothelial cells from microvessels of malignant human gliomas. Int. Immunopharmacol. 2006, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.L.; Grant, N.J.; Temperley, N.D.; Patton, E.E. Small molecule screening in zebrafish: An in vivo approach to identifying new chemical tools and drug leads. Cell Commun. Signal. 2010, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Tobia, C.; Gariano, G.; De Sena, G.; Presta, M. Zebrafish embryo as a tool to study tumor/endothelial cell cross-talk. Biochim. Biophys. Acta 2013, 1832, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, Z.; Tian, H.; Shen, G.; Ma, Y.; Xie, H.; Liu, Y.; Zhao, C.; Deng, S.; Yang, Y.; et al. SKLB1002, a novel potent inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 2011, 17, 4439–4450. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cui, W.; Yu, S.; Xu, C.; Chen, G.; Gu, A.; Li, T.; Cui, Y.; Zhang, X.; Bian, X. A synthetic dl-nordihydroguaiaretic acid (Nordy), inhibits angiogenesis, invasion and proliferation of glioma stem cells within a zebrafish xenotransplantation model. PLoS ONE 2014, 9, e85759. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci. Transl. Med. 2011, 3, 69ra12. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Das, S.; Roy, K.; Chatterjee, M. Overexpression of 5-lipoxygenase and its relation with cell proliferation and angiogenesis in 7,12-dimethylbenz(alpha)anthracene-induced rat mammary carcinogenesis. Mol. Carcinog. 2013, 52, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.N.; Liu, E.S.; Shin, V.Y.; Wu, W.K.; Cho, C.H. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology 2004, 203, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Catalano, A.; Nutini, M.; D’Urbano, E.; Crescenzi, C.; Claria, J.; Libner, R.; Davi, G.; Procopio, A. 5-lipoxygenase regulates malignant mesothelial cell survival: Involvement of vascular endothelial growth factor. FASEB J. 2001, 15, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Vitarelli, E.; Tuccari, G.; Barresi, G. Correlative study of microvessel density and 5-lipoxygenase expression in human sporadic colorectal cancer. Arch. Pathol. Lab. Med. 2008, 132, 1807–1812. [Google Scholar] [PubMed]
- Choi, S.P.; Kim, S.P.; Nam, S.H.; Friedman, M. Antitumor effects of dietary black and brown rice brans in tumor-bearing mice: Relationship to composition. Mol. Nutr. Food Res. 2013, 57, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Feinmark, S.J.; Cannon, P.J. Endothelial cell leukotriene C4 synthesis results from intercellular transfer of leukotriene A4 synthesized by polymorphonuclear leukocytes. J. Biol. Chem. 1986, 261, 16466–16472. [Google Scholar] [PubMed]
- Folco, G.; Murphy, R.C. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006, 58, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Claesson, H.E.; Haeggstrom, J. Human endothelial cells stimulate leukotriene synthesis and convert granulocyte released leukotriene A4 into leukotrienes B4, C4, D4 and E4. Eur. J. Biochem. 1988, 173, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, J.; Malmsten, C.L.; Uden, A.M.; Radmark, O.; Engstedt, L.; Samuelsson, B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood 1981, 58, 658–661. [Google Scholar] [PubMed]
- Tager, A.M.; Bromley, S.K.; Medoff, B.D.; Islam, S.A.; Bercury, S.D.; Friedrich, E.B.; Carafone, A.D.; Gerszten, R.E.; Luster, A.D. Leukotriene B4 receptor BLT1 mediates early effector T-cell recruitment. Nat. Immunol. 2003, 4, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Ott, V.L.; Cambier, J.C.; Kappler, J.; Marrack, P.; Swanson, B.J. Mast cell-dependent migration of effector CD8+ T-cells through production of leukotriene B4. Nat. Immunol. 2003, 4, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Robertson, T.; Smith, J.; Kilfeather, S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am. J. Respir. Crit. Care Med. 2000, 161, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.; Hedqvist, P.; Arfors, K.E. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation 1982, 6, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, S.; Olofsson, A.M.; von Andrian, U.H.; Lundgren-Akerlund, E.; Arfors, K.E. Leukotriene B4-induced neutrophil-mediated endothelial leakage in vitro and in vivo. J. Appl. Physiol. (1985) 1991, 71, 1322–1330. [Google Scholar]
- Di Gennaro, A.; Kenne, E.; Wan, M.; Soehnlein, O.; Lindbom, L.; Haeggstrom, J.Z. Leukotriene B4-induced changes in vascular permeability are mediated by neutrophil release of heparin-binding protein (HBP/CAP37/azurocidin). FASEB J. 2009, 23, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Carnini, C.; Accomazzo, M.R.; Borroni, E.; Vitellaro-Zuccarello, L.; Durand, T.; Folco, G.; Rovati, G.E.; Capra, V.; Sala, A. Synthesis of cysteinyl leukotrienes in human endothelial cells: Subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J. 2011, 25, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.E.; Bochner, B.S.; Undem, B.J. Cysteinyl leukotrienes induce P-selectin expression in human endothelial cells via a non-CysLT1 receptor-mediated mechanism. J. Pharmacol. Exp. Ther. 1997, 281, 655–662. [Google Scholar] [PubMed]
- Zhao, L.; Moos, M.P.; Grabner, R.; Pedrono, F.; Fan, J.; Kaiser, B.; John, N.; Schmidt, S.; Spanbroek, R.; Lotzer, K.; et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat. Med. 2004, 10, 966–973. [Google Scholar] [CrossRef]
- Uzonyi, B.; Lotzer, K.; Jahn, S.; Kramer, C.; Hildner, M.; Bretschneider, E.; Radke, D.; Beer, M.; Vollandt, R.; Evans, J.F.; et al. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 6326–6331. [Google Scholar] [CrossRef] [PubMed]
- Duah, E.; Adapala, R.K.; Al-Azzam, N.; Kondeti, V.; Gombedza, F.; Thodeti, C.K.; Paruchuri, S. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Sci. Rep. 2013, 3, 3274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Wu, N.; Yang, C.S. Leukotriene A4 hydrolase as a target for cancer prevention and therapy. Curr. Cancer Drug Targets 2004, 4, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Claria, J. Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: Implications for cancer therapy. FASEB J. 2003, 17, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Heo, D.S.; Sung, M.W. The shunting of arachidonic acid metabolism to 5-lipoxygenase and cytochrome p450 epoxygenase antagonizes the anti-cancer effect of cyclooxygenase-2 inhibition in head and neck cancer cells. Cell Oncol. 2012, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.N.; Wu, W.K.; Shin, V.Y.; Bruce, I.C.; Wong, B.C.; Cho, C.H. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis 2005, 26, 827–834. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Romero, I.L.; van Houten, B.; Lengyel, E. Adipose tissue and adipocytes supports tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Beales, I.L.P. The role of obesity in oesophageal cancer development. Ther. Adv. Gastroenterol. 2014, 7, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell. Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Horrillo, R.; Gonzalez-Periz, A.; Martinez-Clemente, M.; Lopez-Parra, M.; Ferre, N.; Titos, E.; Moran-Salvador, E.; Deulofeu, R.; Arroyo, V.; Claria, J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J. Immunol. 2010, 184, 3978–3987. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Wada, K.; Murata, Y.; Nakajima, A.; Yamashiro, T.; Kamisaki, Y. Critical role of leukotriene B4 receptor signaling in mouse 3T3-L1 preadipocyte differentiation. Lipids Health Dis. 2013, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oh Da, Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Mayoral, R.; Maris, M.; et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 2015, 21, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Curat, C.A.; Miranville, A.; Sengenes, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumie, A. From blood monocytes to adipose tissue-resident macrophages: Induction of diapedesis by human mature adipocytes. Diabetes 2004, 53, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Kaaman, M.; Ryden, M.; Axelsson, T.; Nordstrom, E.; Sicard, A.; Bouloumie, A.; Langin, D.; Arner, P.; Dahlman, I. ALOX5AP expression, but not gene haplotypes, is associated with obesity and insulin resistance. Int. J. Obes. 2006, 30, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, A.; Hogaboam, C.M.; Luckacs, N.W.; Lincoln, P.M.; Strieter, R.M.; Kunkel, S.L. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: Cross-talk between MCP-1 and leukotriene B4. J. Immunol. 1999, 163, 6148–6154. [Google Scholar] [PubMed]
- Lumeng, C.N.; Maillard, I.; Saltiel, A.R. T-ing up inflammation in fat. Nat. Med. 2009, 15, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Mothe-Satney, I.; Filloux, C.; Amghar, H.; Pons, C.; Bourlier, V.; Galitzky, J.; Grimaldi, P.A.; Feral, C.C.; Bouloumie, A.; van Obberghen, E.; et al. Adipocytes secrete leukotrienes: Contribution to obesity-associated inflammation and insulin resistance in mice. Diabetes 2012, 61, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Hellmann, J.; Tang, Y.; Mathis, S.P.; Kosuri, M.; Bhatnagar, A.; Jala, V.R.; Haribabu, B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J. Immunol. 2011, 187, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.K.; Wen, Y.; Dobrian, A.D.; Cole, B.K.; Ma, Q.; Pei, H.; Williams, M.D.; Bevard, M.H.; Vandenhoff, G.E.; Keller, S.R.; et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E175–E187. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.A. Connecting the dots: Obesity, fatty acids and cancer. Lab. Investig. 2009, 89, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Somasundar, P.; Riggs, D.; Jackson, B.; Vona-Davis, L.; McFadden, D.W. Leptin stimulates esophageal adenocarcinoma growth by nonapoptotic mechanisms. Am. J. Surg. 2003, 186, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Mensing, H.; Czarnetzki, B.M. Leukotriene B4 induces in vitro fibroblast chemotaxis. J. Investig. Dermatol. 1984, 82, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Chibana, K.; Ishii, Y.; Asakura, T.; Fukuda, T. Up-regulation of cysteinyl leukotriene 1 receptor by IL-13 enables human lung fibroblasts to respond to leukotriene C4 and produce eotaxin. J. Immunol. 2003, 170, 4290–4295. [Google Scholar] [CrossRef] [PubMed]
- Eap, R.; Jacques, E.; Semlali, A.; Plante, S.; Chakir, J. Cysteinyl leukotrienes regulate TGF-beta(1) and collagen production by bronchial fibroblasts obtained from asthmatic subjects. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.F.; Barrios, C.; Funk, C.D.; Larsson, O.; Haeggstrom, J.; Radmark, O. Human fibroblasts show expression of the leukotriene-A4-hydrolase gene, which is increased after simian-virus-40 transformation. Eur. J. Biochem. 1990, 191, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Kondo, M.; Nagai, A. Effects of a leukotriene B4 receptor antagonist on bleomycin-induced pulmonary fibrosis. Eur. Respir. J. 2009, 34, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- El-Swefy, S.; Hassanen, S.I. Improvement of hepatic fibrosis by leukotriene inhibition in cholestatic rats. Ann. Hepatol. 2009, 8, 41–49. [Google Scholar] [PubMed]
- Taylor, P.M.; Woodfield, R.J.; Hodgkin, M.N.; Pettitt, T.R.; Martin, A.; Kerr, D.J.; Wakelam, M.J. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A(2) and 5-lipoxygenase catalyzed pathway. Oncogene 2002, 21, 5765–5772. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, G.Y.; Pidgeon, G.P. Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway. Int. J. Mol. Sci. 2017, 18, 236. https://doi.org/10.3390/ijms18020236
Moore GY, Pidgeon GP. Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway. International Journal of Molecular Sciences. 2017; 18(2):236. https://doi.org/10.3390/ijms18020236
Chicago/Turabian StyleMoore, Gillian Y., and Graham P. Pidgeon. 2017. "Cross-Talk between Cancer Cells and the Tumour Microenvironment: The Role of the 5-Lipoxygenase Pathway" International Journal of Molecular Sciences 18, no. 2: 236. https://doi.org/10.3390/ijms18020236