Emerging Roles of IL-33/ST2 Axis in Renal Diseases
Abstract
:1. Introduction
1.1. Interleukin-33 and ST2 Signaling
1.2. Distribution of IL-33 and ST2 in the Kidney
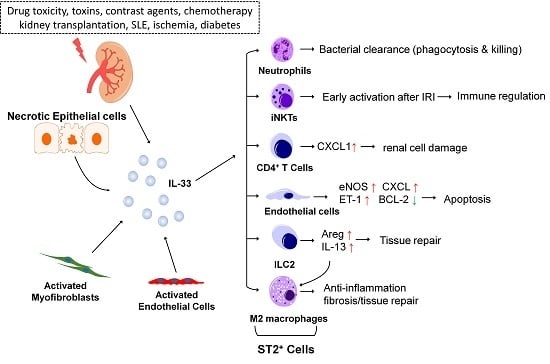
2. IL-33 in Chronic Kidney Injury
2.1. IL-33 and Chronic Kidney Disease (CKD)
2.2. IL-33 and Diabetic Nephropathy
2.3. IL-33 and Systemic Lupus Erythematosus (SLE) and Lupus Nephritis
2.4. IL-33 in Renal Cell Carcinoma
3. IL-33 in Acute Kidney Injury
3.1. IL-33 and Acute Kidney Injury (AKI)
3.2. IL-33 and Obstructive Renal Injury
3.3. IL-33-Mediated ILC2 Expansion in Acute Renal Injury
3.4. IL-33 and Acute Renal Injury Associated with Infection
4. IL-33 in Renal Transplantation
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rewa, O.; Bagshaw, S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Cardiovascular disease in chronic renal failure: Pathophysiologic aspects. Semin. Dial. 2003, 16, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Balla, S.; Nusair, M.B.; Alpert, M.A. Risk factors for atherosclerosis in patients with chronic kidney disease: Recognition and management. Curr. Opin. Pharmacol. 2013, 13, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Panzer, U.; Anders, H.J.; Rees, A.J. The immune system and kidney disease: Basic concepts and clinical implications. Nat. Rev. Immunol. 2013, 13, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K. Role of oxidative stress in drug-induced kidney injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef] [PubMed]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 743–750. [Google Scholar] [PubMed]
- Umbro, I.; Gentile, G.; Tinti, F.; Muiesan, P.; Mitterhofer, A.P. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J. Infect. 2016, 72, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Barma, S.; Konwar, N.; Dewanjee, S.; Manna, P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur. J. Pharmacol. 2016, 791, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Baekkevold, E.S.; Roussigne, M.; Yamanaka, T.; Johansen, F.E.; Jahnsen, F.L.; Amalric, F.; Brandtzaeg, P.; Erard, M.; Haraldsen, G.; Girard, J.P. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 2003, 163, 69–79. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel IL-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, M.; Volarevic, V.; Radosavljevic, G.; Jovanovic, I.; Pejnovic, N.; Arsenijevic, N.; Lukic, M.L. IL-33/ST2 axis in inflammation and immunopathology. Immunol. Res. 2012, 52, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Hei, H.; Dobner, S.; Lee, R.T. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 2012, 287, 6941–6948. [Google Scholar] [CrossRef] [PubMed]
- Iwahana, H.; Yanagisawa, K.; Ito-Kosaka, A.; Kuroiwa, K.; Tago, K.; Komatsu, N.; Katashima, R.; Itakura, M.; Tominaga, S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur. J. Biochem. 1999, 264, 397–406. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; MacDonald, T.T.; Hermoso, M.A. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015, 26, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel “alarmin”? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.I.; Guihard, P.; Danger, Y.; Noel, G.; Le Seyec, J.; Boutet, M.A.; Richards, C.D.; L’Helgoualc’h, A.; Genet, V.; Lucas-Clerc, C.; et al. Oncostatin M induces IL-33 expression in liver endothelial cells in mice and expands ST2+CD4+ lymphocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G542–G553. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249–7254. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A.; Nguyen, Q.; He, Z.; Turkmen, K.; Won Lee, D.; Hernando, A.A.; Altmann, C.; Toker, A.; Pacic, A.; Ljubanovic, D.G.; et al. IL-33 exacerbates acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Luthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Roussel, L.; Erard, M.; Cayrol, C.; Girard, J.P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008, 9, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Gautier, V.; Cayrol, C.; Farache, D.; Roga, S.; Monsarrat, B.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Girard, J.P. Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells. Sci. Rep. 2016, 6, 34255. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mohs, A.; Thomas, M.; Klare, J.; Ross, R.; Schmitz, M.L.; Martin, M.U. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J. Immunol. 2011, 187, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tao, L.; Chen, C.; Song, H.; Li, Z.; Gao, Y.; Nie, J.; Piccioni, M.; Shi, G.; Li, B. The deubiquitinase USP17 regulates the stability and nuclear function of IL-33. Int. J. Mol. Sci. 2015, 16, 27956–27966. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.; Smith, D.E.; Dekruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Muto, T.; Kawagoe, T.; Matsumoto, M.; Sasaki, Y.; Matsushita, K.; Taki, Y.; Futatsugi-Yumikura, S.; Tsutsui, H.; Ishii, K.J.; et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. USA 2012, 109, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.S.; Scott, I.C.; Majithiya, J.B.; Rapley, L.; Kemp, B.P.; England, E.; Rees, D.G.; Overed-Sayer, C.L.; Woods, J.; Bond, N.J.; et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 2015, 6, 8327. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Meyer, C.A.; de Vera Mudry, M.C.; Schlicht, S.; Smith, S.H.; Iglesias, A.; Cote-Sierra, J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J. Autoimmun. 2014, 55, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, D.R.; Ramirez-Carrozzi, V.; Choy, D.F.; Lee, K.; Soriano, R.; Jia, G.; Abbas, A.R.; Modrusan, Z.; Pappu, R.; Arron, J.R. IL-13 mediates IL-33-dependent mast cell and type 2 innate lymphoid cell effects on bronchial epithelial cells. J. Allergy Clin. Immunol. 2015, 136, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, Y.J.; Subramanian, S.; Lee, H.H.; Albacker, L.A.; Matangkasombut, P.; Savage, P.B.; McKenzie, A.N.; Smith, D.E.; Rottman, J.B.; et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012, 129, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, P.; Duan, L.; Yang, L.; Wang, J. IL-33 and kidney disease (Review). Mol. Med. Rep. 2016, 13, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guabiraba, R.; Besnard, A.G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chang, Y.J.; Su, C.H.; Tsai, T.H.; Chen, S.D.; Hsing, C.H.; Yang, J.L. Upregulation of Interleukin-33 in obstructive renal injury. Biochem. Biophys. Res. Commun. 2016, 473, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Shelite, T.R.; Liang, Y.; Wang, H.; Mendell, N.L.; Trent, B.J.; Sun, J.; Gong, B.; Xu, G.; Hu, H.; Bouyer, D.H.; et al. IL-33-dependent endothelial activation contributes to apoptosis and renal injury in orientia tsutsugamushi-infected mice. PLoS Negl. Trop. Dis. 2016, 10, e0004467. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.G.; Kim, H.J.; Kim, J.; Kang, S.W.; Moon, U.J.; Cho, H.R.; Kwon, B. IL-33 enhances host tolerance to candida albicans kidney infections through induction of IL-13 production by CD4+ T cells. J. Immunol. 2015, 194, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Niu, Z.; Tan, J.; Yang, J.; Liu, Y.; Ma, H.; Lee, V.W.; Sun, S.; Song, X.; Guo, M.; et al. IL-25 elicits innate lymphoid cells and multipotent progenitor type 2 cells that reduce renal ischemic/reperfusion injury. J. Am. Soc. Nephrol. 2015, 26, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Riedel, J.H.; Becker, M.; Kopp, K.; Duster, M.; Brix, S.R.; Meyer-Schwesinger, C.; Kluth, L.A.; Gnirck, A.C.; Attar, M.; Krohn, S.; et al. IL-33-mediated expansion of type 2 innate lymphoid cells protects from progressive glomerulosclerosis. J. Am. Soc. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ramadan, A.M.; Griesenauer, B.; Li, W.; Turner, M.J.; Liu, C.; Kapur, R.; Hanenberg, H.; Blazar, B.R.; Tawara, I.; et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.A.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Groger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell. Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Delrue, L.; van Durme, F.; Muller, O.; Casselman, F.; de Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Bjornsson, T.D. Use of serum creatinine concentrations to determine renal function. Clin. Pharmacokinet. 1979, 4, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Betensky, R.A.; Bonventre, J.V. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol. Dial. Transplant. 2009, 24, 3263–3265. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004, 30, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.S.; Na, S.P.; Zhang, P.; Jia, X.B.; Liu, R.C.; Yu, C.Y.; Mu, S.H.; Xie, R.J. Characterization of interleukin-33 and soluble ST2 in serum and their association with disease severity in patients with chronic kidney disease. J. Clin. Immunol. 2012, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Caner, S.; Usluogullari, C.A.; Balkan, F.; Buyukcam, F.; Kaya, C.; Sacikara, M.; Koca, C.; Ersoy, R.; Cakir, B. Is IL-33 useful to detect early stage of renal failure? Renal Fail. 2014, 36, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Russo, S.; Bonanno, A.; Innao, V.; Gangemi, S. Reduced IL-33 plasma levels in multiple myeloma patients are associated with more advanced stage of disease. Br. J. Haematol. 2013, 160, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Huang, Y.; Su, Q.; Lin, Q.; Liu, W.; Luo, J.; Yu, B.; He, Y.; Qian, H.; Liu, Y.; et al. Potential of IL-33 for preventing the kidney injury via regulating the lipid metabolism in gout patients. J. Diabetes Res. 2016, 2016, 1028401. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K.; Tesch, G.H. Inflammation in diabetic nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.K.; Chong, T.W.; Loh, H.L.; Lim, K.H.; Gan, V.H.; Wang, M.; Kon, O.L. Negative regulatory responses to metabolically triggered inflammation impair renal epithelial immunity in diabetes mellitus. J. Mol. Med. 2013, 91, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Caporali, A.; Meloni, M.; Miller, A.M.; Vierlinger, K.; Cardinali, A.; Spinetti, G.; Nailor, A.; Faglia, E.; Losa, S.; Gotti, A.; et al. Soluble ST2 is regulated by p75 neurotrophin receptor and predicts mortality in diabetic patients with critical limb ischemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e149–e160. [Google Scholar] [CrossRef] [PubMed]
- Ryba-Stanislawowska, M.; Werner, P.; Skrzypkowska, M.; Brandt, A.; Mysliwska, J. IL-33 effect on quantitative changes of CD4+CD25highFOXP3+ regulatory T cells in children with type 1 diabetes. Mediat. Inflamm. 2016, 2016, 9429760. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Purves, D.; McConnachie, A.; Asquith, D.L.; Batty, G.D.; Burns, H.; Cavanagh, J.; Ford, I.; McLean, J.S.; Packard, C.J.; et al. Soluble ST2 associates with diabetes but not established cardiovascular risk factors: A new inflammatory pathway of relevance to diabetes? PLoS ONE 2012, 7, e47830. [Google Scholar] [CrossRef] [PubMed]
- Calvin, A.D.; Misra, S.; Pflueger, A. Contrast-induced acute kidney injury and diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Onk, D.; Onk, O.A.; Turkmen, K.; Erol, H.S.; Ayazoglu, T.A.; Keles, O.N.; Halici, M.; Topal, E. Melatonin attenuates contrast-induced nephropathy in diabetic rats: The role of Interleukin-33 and oxidative stress. Mediat. Inflamm. 2016, 2016, 9050828. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, L.; Turkmen, K.; Kandemir, F.M.; Ozkaraca, M.; Kucukler, S.; Gurbuzel, M.; Comakli, S. The possible role of interleukin-33 as a new player in the pathogenesis of contrast-induced nephropathy in diabetic rats. Ren. Fail. 2016, 38, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, E.V.; la Cava, A. Cytokines in systemic lupus erythematosus. Curr. Mol. Med. 2009, 9, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Koutsokeras, T.; Healy, T. Systemic lupus erythematosus and lupus nephritis. Nat. Rev. Drug Discov. 2014, 13, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Appel, G.B. Updates on the treatment of lupus nephritis. J. Am. Soc. Nephrol. JASN 2010, 21, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Law, H.K. The Role of Autophagy in Lupus Nephritis. Int. J. Mol. Sci. 2015, 16, 25154–25167. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.L.; Wong, C.K.; Tam, L.S. The alarmin functions of high-mobility group box-1 and IL-33 in the pathogenesis of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2013, 9, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, W.; Zheng, X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation 2014, 37, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, Y.; Xi, W.; Li, C.; Zhong, R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin. Exp. Med. 2011, 11, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.Y.; Huang, F.P.; Ip, W.K.; Lo, Y.; Wong, F.Y.; Chan, E.Y.; Lam, K.F.; Xu, D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology 2010, 49, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, L.; Chang, Y.; Zhou, L.; Fu, H.; Zhang, W.; Yang, Y.; Xu, J. IL-33 is associated with unfavorable postoperative survival of patients with clear-cell renal cell carcinoma. Tumour Biol. 2016, 37, 11127–11134. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Shinn, H.K.; Kang, J.H.; Na, W.J.; Kim, Y.H.; Park, C.S. Anti-interleukin-33 reduces ovalbumin-induced nephrotoxicity and expression of kidney injury molecule-1. Int. Neurourol. J. 2016, 20, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Nino, M.D.; Ruiz Ortega, M.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. JASN 2017, 28, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gulcin, I. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A multi-biomarker approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.S.; Thornhill, B.A.; Minor, J.J.; Gordon, K.A.; Galarreta, C.I.; Chevalier, R.L. Fight-or-flight: Murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am. J. Physiol. Ren. Physiol. 2012, 303, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 2011, 186, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Hardman, C.; Ogg, G. Interleukin-33, friend and foe in type-2 immune responses. Curr. Opin. Immunol. 2016, 42, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanth, C.L.; Jacob, S.P.; Chaithra, V.H.; de Castro-Faria-Neto, H.C.; Marathe, G.K. Sepsis: In search of cure. Inflamm. Res. 2016, 65, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turnquist, H.R.; Hoffman, R.; Billiar, T.R. Role of the IL-33-ST2 axis in sepsis. Mil. Med. Res. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Cekmez, F.; Fidanci, M.K.; Ayar, G.; Saldir, M.; Karaoglu, A.; Gunduz, R.C.; Tunc, T.; Kalkan, G. Diagnostic value of upar, IL-33, and ST2 levels in childhood sepsis. Clin. Lab. 2016, 62, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Krenn, C.; Roth, G.; Moser, B.; Dworschak, M.; Jensen-Jarolim, E.; Spittler, A.; Sautner, T.; Bonaros, N.; Wolner, E.; et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004, 30, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Hoogerwerf, J.J.; Tanck, M.W.; van Zoelen, M.A.; Wittebole, X.; Laterre, P.F.; van der Poll, T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010, 36, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Parenica, J.; Malaska, J.; Jarkovsky, J.; Lipkova, J.; Dastych, M.; Helanova, K.; Litzman, J.; Tomandl, J.; Littnerova, S.; Sevcikova, J.; et al. Soluble ST2 levels in patients with cardiogenic and septic shock are not predictors of mortality. Exp. Clin. Cardiol. 2012, 17, 205–209. [Google Scholar] [PubMed]
- Attur, R.P.; Kuppasamy, S.; Bairy, M.; Nagaraju, S.P.; Pammidi, N.R.; Kamath, V.; Kamath, A.; Rao, L.; Bairy, I. Acute kidney injury in scrub typhus. Clin. Exp. Nephrol. 2013, 17, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Tran, V.G.; Kim, W.; Kim, J.; Cho, H.R.; Kwon, B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-candida albicans response by modulating TLR and dectin-1 signals. J. Immunol. 2012, 189, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Giraud, S.; Robin, A.; Barra, A.; Bridoux, F.; Ameteau, V.; Hauet, T.; Girard, J.P.; Touchard, G.; Gombert, J.M.; et al. The alarmin concept applied to human renal transplantation: Evidence for a differential implication of HMGB1 and IL-33. PLoS ONE 2014, 9, e88742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Xu, Z.; Han, Z.; Tao, J.; Lu, P.; Huang, Z.; Zhou, W.; Zhao, C.; Tan, R.; et al. The potential role of IL-33 in renal transplant recipients with chronic allograft dysfunction. Ann. Transpl. 2016, 21, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Mansell, H.; Soliman, M.; Elmoselhi, H.; Shoker, A. Elevated circulating Interleukin 33 levels in stable renal transplant recipients at high risk for cardiovascular events. PLoS ONE 2015, 10, e0142141. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, S.; Le Berre, L.; Herve, C.; Valanciute, A.; Kamal, M.; Naulet, J.; Tesson, L.; Foucher, Y.; Soulillou, J.P.; Sahali, D.; et al. Potential role of soluble ST2 protein in idiopathic nephrotic syndrome recurrence following kidney transplantation. Am. J. Kidney Dis. 2009, 54, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Vander Lugt, M.T.; Braun, T.M.; Hanash, S.; Ritz, J.; Ho, V.T.; Antin, J.H.; Zhang, Q.; Wong, C.H.; Wang, H.; Chin, A.; et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N. Engl. J. Med. 2013, 369, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, D.K.; Schwarze, V.; Matta, B.M.; Tkachev, V.; Lieberknecht, E.; Liu, Q.; Koehn, B.H.; Pfeifer, D.; Taylor, P.A.; Prinz, G.; et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood 2015, 125, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.M.; Reichenbach, D.K.; Zhang, X.; Mathews, L.; Koehn, B.H.; Dwyer, G.K.; Lott, J.M.; Uhl, F.M.; Pfeifer, D.; Feser, C.J.; et al. Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood 2016, 128, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Vestergaard, C.; Johansen, C.; Deleuran, M.; Hvid, M. Measuring serum concentrations of interleukin-33 in atopic dermatitis is associated with potential false positive results. Springerplus 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Ketelaar, M.E.; Nawijn, M.C.; Shaw, D.E.; Koppelman, G.H.; Sayers, I. The challenge of measuring IL-33 in serum using commercial ELISA: Lessons from asthma. Clin. Exp. Allergy 2016, 46, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Riviere, E.; Ly, B.; Boudaoud, S.; Chavez, H.; Nocturne, G.; Chanson, P.; Miceli Richard, C.; Mariette, X. Pitfalls for detecting interleukin-33 by ELISA in the serum of patients with primary Sjogren syndrome: Comparison of different kits. Ann. Rheum. Dis. 2016, 75, 633–635. [Google Scholar] [CrossRef] [PubMed]

| Kidney Diseases | Roles of IL-33/ST2 Axis | IL-33 Levels | sST2 Levels | Species | Reference |
|---|---|---|---|---|---|
| Chronic renal failure |
| (−) Serum | (↑) Serum | human | [58] |
| |||||
| (−) Serum | human | [59] | ||
| Diabetic nephritis |
| (↑) Kidney mRNA | [63] | ||
| (↑) Serum | human | [64] | ||
| (↑) Serum | human | [65] | ||
| Contrast-induced DN |
| (↑) Kidney protein | rats | [68,69] | |
| (↑) Serum | |||||
| Multiple myeloma patients with kidney failure |
| (−) Serum | human | [60] | |
| |||||
| Gout |
| (↑) Serum | human | [61] | |
| |||||
| Renal cell carcinoma |
| (↑) Tissue | [79] | ||
| SLE |
| (↑) Serum | (↑) Serum | human mice | [76,77] |
| mice | [76] | |||
| (−) Serum | (↑) Serum | human | [78] | |
| UUO |
| (↑) Kidney mRNA | (↑) Kidney mRNA | mice | [46] |
| (↑) Kidney Protein | ||||
| Ischemia/Reperfusion renal injury |
| mice | [49] | ||
| Renal Transplantation |
| (↑) Urine | (↑) Urine | human | [95] |
| (↑) Serum | (↑) Serum | |||
| (↑) Serum | human | [96] | ||
| (↑) Serum | human | [97] | ||
| (↑) Serum | human | [98] | ||
| GVHD |
| (↑) Serum | human | [99] | |
| (↑) Serum | mice | [100] | ||
| (↑) Serum | mice | [51] | ||
| mice | [101] | |||
| Drug/toxin-induced AKI | |||||
| Cisplatin |
| (↑) Kidney protein | mice | [27] | |
| (↑) Serum | ||||
| |||||
| OVA |
| mice | [80] | ||
| Folic acid |
| (↑) Kidney protein | mice | [81] | |
| (↑) Serum | |||||
| Paracetamol |
| (↑) Kidney protein | rats | [82] | |
| Adriamycin |
| mice | [50] | ||
| |||||
| Infection-associated kidney injury | |||||
| Orientia tsutsugamushi |
| (↑) Kidney mRNA | (↑) Kidney mRNA | mice | [47] |
| (↑) Liver mRNA | (↑) Liver mRNA | |||
| |||||
| Candida albicans |
| mice | [48] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-Y.; Li, L.-C.; Yang, J.-L. Emerging Roles of IL-33/ST2 Axis in Renal Diseases. Int. J. Mol. Sci. 2017, 18, 783. https://doi.org/10.3390/ijms18040783
Chen W-Y, Li L-C, Yang J-L. Emerging Roles of IL-33/ST2 Axis in Renal Diseases. International Journal of Molecular Sciences. 2017; 18(4):783. https://doi.org/10.3390/ijms18040783
Chicago/Turabian StyleChen, Wei-Yu, Lung-Chih Li, and Jenq-Lin Yang. 2017. "Emerging Roles of IL-33/ST2 Axis in Renal Diseases" International Journal of Molecular Sciences 18, no. 4: 783. https://doi.org/10.3390/ijms18040783