Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes
Abstract
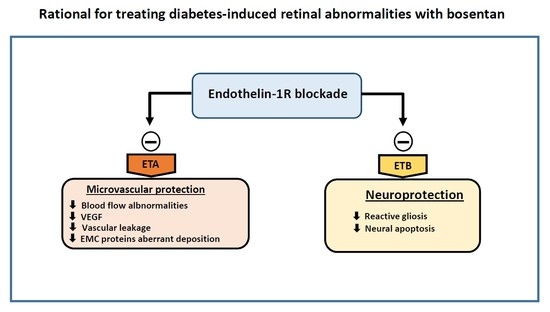
:1. Introduction
2. Results
2.1. Endothelin-1 And Its Receptors A (ETA) And B (ETB) Were Upregulated in the Retina of Diabetic Donors
2.2. Bosentan Ameliorated the Upregulation of Endothelin-1 And Its Receptors A (ETA) And B (ETB) in the Retinas of Diabetic Mice
2.3. Bosentan Prevented Diabetes-Induced Neurodegeneration in Diabetic Mice
2.4. Glial Activation
2.5. Retinal Apoptosis
2.6. Bosentan Decreased PKC-β, TNF-α And VEGF Upregulation Induced by Diabetes
2.6.1. In Vivo Studies
2.6.2. In Vitro Studies
2.7. Pharmacokinetics
3. Discussion
4. Materials Material and Methods
4.1. Human Retinas
4.2. Animals
4.3. Immunohistochemistry for Endothelin (ET-1) And Endothelin Receptors (ETA-R and ETB-R)
4.4. Neurodegeneration Measurements
4.4.1. Measurements of Glial Activation
4.4.2. Immunohistochemical Analysis for Apoptosis Assessment
4.4.3. Other Immunohistochemical Analysis
4.4.4. Pharmacokinetic Analyses
4.5. In Vitro Studies in Human Retinal Endothelial Cells
4.5.1. Measurement of HREC Permeability
4.5.2. RNA Extraction and Quantitative Real-Time PCR
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Gardner, T.W. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.; Evans, T.; Mukherjee, K.; Downey, D.; Chakrabarti, S. Diabetes-induced vascular dysfunction in the retina: Role of endothelins. Diabetologia 1999, 42, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.; Deng, D.X.; Chen, S.; Chakrabarti, S. Endothelin receptor blockade prevents augmented extracellular matrix component mRNA expression and capillary basement membrane thickening in the retina of diabetic and galactose-fed rats. Diabetes 2000, 49, 662–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuzawa, K.; Jesmin, S.; Maeda, S.; Zaedi, S.; Shimojo, N.; Miyauchi, T.; Goto, K. Effect of endothelin dual receptor antagonist on VEGF levels in streptozotocin-induced diabetic rat retina. Exp. Biol. Med. 2006, 231, 1090–1094. [Google Scholar]
- Masuzawa, K.; Goto, K.; Jesmin, S.; Maeda, S.; Miyauchi, T.; Kaji, Y.; Oshika, T.; Hori, S. An endothelin type A receptor antagonist reverses upregulated VEGF and ICAM-1 levels in streptozotocin-induced diabetic rat retina. Curr. Eye Res. 2006, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yadav, A.S.; Leskova, W.; Harris, N.R. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp. Eye Res. 2010, 91, 670–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüscher, T.F.; Barton, M. Endothelins and endothelin receptor antagonists: Therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000, 102, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.Z.; Phatak, N.R.; Stankowska, D.L.; He, S.; Ma, H.-Y.; Mueller, B.H.; Jiang, M.; Luedtke, R.; Yang, S.; Brownlee, C.; et al. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.R.; Rao, V.R.; Dauphin, R.; Prasanna, G.; Johnson, C.; Yorio, T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can. J. Physiol. Pharmacol. 2008, 86, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Tonari, M.; Kurimoto, T.; Horie, T.; Sugiyama, T.; Ikeda, T.; Oku, H. Blocking endothelin-B receptors rescues retinal ganglion cells from optic nerve injury through suppression of neuroinflammation. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3490–3500. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.G.; Boden, J.P.; Biecker, E.; Reichen, J.; Rothen, B. Endothelin antagonism prevents diabetic retinopathy in NOD mice: A potential role of the angiogenic factor adrenomedullin. Exp. Biol. Med. 2006, 231, 1101–1105. [Google Scholar]
- De Juan, J.A.; Moya, F.J.; Ripodas, A.; Bernal, R.; Fernandez-Cruz, A.; Fernandez-Durango, R. Changes in the density and localisation of endothelin receptors in the early stages of rat diabetic retinopathy and the effect of insulin treatment. Diabetologia 2000, 43, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, S.; Gan, X.T.; Merry, A.; Karmazyn, M.; Sima, A.A. Augmented retinal endothelin-1, endothelin-3, endothelinA and endothelinB gene expression in chronic diabetes. Curr. Eye Res. 1998, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Sampedro, J.; Valeri, M.; Garcia-Ramirez, M.; Pasquali, C.; Simó, R. Calcium Dobesilate Prevents Neurodegeneration and Vascular Leakage in Experimental Diabetes. Curr. Eye Res. 2017, 42, 1273–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCumber, M.W.; D’Anna, S.A. Endothelin receptor-binding subtypes in the human retina and choroid. Arch. Ophthalmol. 1994, 112, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M. The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc. Health Risk Manag. 2008, 4, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pallarés, M.; Rollín, R.; Martínez-Montero, J.C.; Fernández-Cruz, A.; Bravo-Llata, C.; Fernández-Durango, R. Immunoreactive endothelin-1 in the vitreous humor and epiretinal membranes of patients with proliferative diabetic retinopathy. Retina 2007, 27, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Khuu, L.-A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous humor endothelin-1 and total retinal blood flow in patients with non-proliferative diabetic retinopathy. Eye 2017, 31, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.C.; Rollins, S.D.; Ye, M.; Batlle, D.; Fawzi, A.A. Endothelin receptor-A antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Invest. Ophthalmol. Vis. Sci. 2014, 55, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Takahara, N.; Gabriele, A.; Chou, E.; Naruse, K.; Suzuma, K.; Yamauchi, T.; Ha, S.W.; Meier, M.; Rhodes, C.J.; et al. Induction of endothelin-1 expression by glucose: An effect of protein kinase C activation. Diabetes 2000, 49, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. The potential role of PKC beta in diabetic retinopathy and macular edema. Surv. Ophthalmol. 2002, 47, 263–269. [Google Scholar] [CrossRef]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrósio, A.F.; Antonetti, D.A. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Watts, H.; Hille, C.; Philpott, K.; Clark, P.; Gentleman, M.C.S.; Jen, L.-S. Glial and endothelial blood-retinal barrier responses to amyloid-beta in the neural retina of the rat. Clin. Ophthalmol. 2008, 2, 801–816. [Google Scholar] [CrossRef] [PubMed]







| Groups | Dose Regimen | Time-Points Post Dose | Animal Number |
|---|---|---|---|
| 1 | Single 50 µL instillation in right eye | 0.25 h | 5 |
| 2 | 0.5 h | 5 | |
| 3 | 1 h | 5 | |
| 4 | 2 h | 5 | |
| 5 | 4 h | 5 | |
| 6 | 8 h | 5 | |
| 7 | 24 h | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, P.; Simó-Servat, O.; Sampedro, J.; Solà-Adell, C.; Garcia-Ramírez, M.; Ramos, H.; Guerrero, M.; Suñé-Negre, J.M.; Ticó, J.R.; Montoro, B.; et al. Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes. Int. J. Mol. Sci. 2018, 19, 3578. https://doi.org/10.3390/ijms19113578
Bogdanov P, Simó-Servat O, Sampedro J, Solà-Adell C, Garcia-Ramírez M, Ramos H, Guerrero M, Suñé-Negre JM, Ticó JR, Montoro B, et al. Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes. International Journal of Molecular Sciences. 2018; 19(11):3578. https://doi.org/10.3390/ijms19113578
Chicago/Turabian StyleBogdanov, Patricia, Olga Simó-Servat, Joel Sampedro, Cristina Solà-Adell, Marta Garcia-Ramírez, Hugo Ramos, Marta Guerrero, Josep Maria Suñé-Negre, Josep Ramon Ticó, Bruno Montoro, and et al. 2018. "Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes" International Journal of Molecular Sciences 19, no. 11: 3578. https://doi.org/10.3390/ijms19113578