Modifiable Individual Risks of Perioperative Blood Transfusions and Acute Postoperative Complications in Total Hip and Knee Arthroplasty
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Outcome Measures
2.3. Statistical Analysis
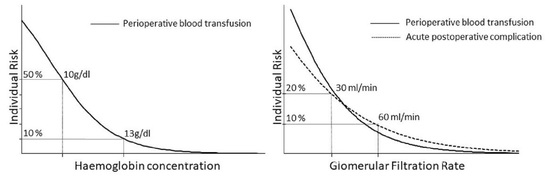
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitterecker, A.; Hofmann, A.; Trentino, K.M.; Lloyd, A.; Leahy, M.F.; Schwarzbauer, K.; Tschoellitsch, T.; Böck, C.; Hochreiter, S.; Meier, J. Machine learning-based prediction of transfusion. Transfusion 2020, 60, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Devana, S.K.; Lee, C.; Kianian, R.; van der Schaar, M.; SooHoo, N.F. Development of a Novel, Potentially Universal Machine Learning Algorithm for Prediction of Complications After Total Hip Arthroplasty. J. Arthroplast. 2021, 36, 1655–1662.e1651. [Google Scholar] [CrossRef] [PubMed]
- Grosso, M.J.; Boddapati, V.; Cooper, H.J.; Geller, J.A.; Shah, R.P.; Neuwirth, A.L. The Effect of Preoperative Anemia on Complications after Total Hip Arthroplasty. J. Arthroplast. 2020, 35, S214–S218. [Google Scholar] [CrossRef]
- Loftus, T.J.; Spratling, L.; Stone, B.A.; Xiao, L.; Jacofsky, D.J. A Patient Blood Management Program in Prosthetic Joint Arthroplasty Decreases Blood Use and Improves Outcomes. J. Arthroplast. 2016, 31, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Luangwaranyoo, A.; Suksintharanon, M.; Tangadulrat, P.; Iamthanaporn, K.; Hongnaparak, T.; Yuenyongviwat, V. Factors for Blood Transfusions Following Hemi Hip Arthroplasty for Patients With Femoral Neck Fracture. Geriatr. Orthop. Surg. Rehabil. 2020, 11. [Google Scholar] [CrossRef]
- Yeh, J.Z.; Chen, J.Y.; Bin Abd Razak, H.R.; Loh, B.H.; Hao, Y.; Yew, A.K.; Chia, S.L.; Lo, N.N.; Yeo, S.J. Preoperative haemoglobin cut-off values for the prediction of post-operative transfusion in total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 3293–3298. [Google Scholar] [CrossRef]
- Maempel, J.F.; Wickramasinghe, N.R.; Clement, N.D.; Brenkel, I.J.; Walmsley, P.J. The pre-operative levels of haemoglobin in the blood can be used to predict the risk of allogeneic blood transfusion after total knee arthroplasty. Bone Jt. J. 2016, 98-B, 490–497. [Google Scholar] [CrossRef]
- Moreau, P. Minimally invasive total hip arthroplasty using Hueter’s direct anterior approach. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2018, 28, 771–779. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [Green Version]
- Owens, J.; Otero, J.E.; Noiseux, N.O.; Springer, B.D.; Martin, J.R. Risk Factors for Post-Operative Blood Transfusion Following Total Knee Arthroplasty. Iowa Orthop. J. 2020, 40, 69–73. [Google Scholar]
- Nolan, H.R.; Davenport, D.L.; Ramaiah, C. BMI Is an Independent Preoperative Predictor of Intraoperative Transfusion and Postoperative Chest-Tube Output. Int. J. Angiol. 2013, 22, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Lemmens, H.J.; Bernstein, D.P.; Brodsky, J.B. Estimating blood volume in obese and morbidly obese patients. Obes. Surg. 2006, 16, 773–776. [Google Scholar] [CrossRef]
- Sizer, S.C.; Cherian, J.J.; Elmallah, R.D.; Pierce, T.P.; Beaver, W.B.; Mont, M.A. Predicting Blood Loss in Total Knee and Hip Arthroplasty. Orthop. Clin. N. Am. 2015, 46, 445–459. [Google Scholar] [CrossRef]
- Kral, J.G. Morbidity of severe obesity. Surg. Clin. N. Am. 2001, 81, 1039–1061. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.I.; Choi, W.; Kim, T.W.; Lee, Y.S. Effectiveness of iron supplementation in the perioperative management of total knee arthroplasty: A systematic review. Knee Surg. Relat. Res. 2020, 32, 44. [Google Scholar] [CrossRef]
- Scrimshire, A.B.; Booth, A.; Fairhurst, C.; Kotze, A.; Reed, M.; McDaid, C. Preoperative iron treatment in anaemic patients undergoing elective total hip or knee arthroplasty: A systematic review and meta-analysis. BMJ Open 2020, 10, e036592. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Gracia, C.; Mateo-Agudo, J.; Herrera, A.; Munoz, M. On the relevance of preoperative haemoglobin optimisation within a Patient Blood Management programme for elective hip arthroplasty surgery. Blood Transfus. Trasfus. Sangue 2020, 18, 182–190. [Google Scholar] [CrossRef]
- Bedair, H.; Yang, J.; Dwyer, M.K.; McCarthy, J.C. Preoperative erythropoietin alpha reduces postoperative transfusions in THA and TKA but may not be cost-effective. Clin. Orthop. Relat. Res. 2015, 473, 590–596. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, T.; Kon, E.; Martorelli, F.; Monteleone, G.; Simili, V.; Tasso, F.; Di Matteo, B.; Scardino, M. Effect of intravenous ferric carboxymaltose supplementation in non-anaemic iron deficient patients undergoing hip and knee arthroplasty. J. Biol. Regul. Homeost. Agents 2020, 34, 69–77. [Google Scholar] [PubMed]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization (WHO/NMH/NHD/MNM/11.1). 2011. Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 8 October 2021).
- Munting, K.E.; Klein, A.A. Optimisation of pre-operative anaemia in patients before elective major surgery—Why, who, when and how? Anaesthesia 2019, 74 (Suppl. 1), 49–57. [Google Scholar] [CrossRef]
- Kaiser, C.; Tillmann, F.P.; Lochter, J.; Landgraeber, S.; Jager, M. The influence of chronic kidney disease on the duration of hospitalisation and transfusion rate after elective hip and knee arthroplasty. Int. Urol. Nephrol. 2019, 51, 147–153. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, H.; Tanaka, A.; Yasui, M.; Nishihira, J.; Murayama, N. Effect of Increased Daily Water Intake and Hydration on Health in Japanese Adults. Nutrients 2020, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Kim, J.S.; Jo, M.J.; Cho, E.J.; Ahn, S.Y.; Ko, G.J.; Kwon, Y.J.; Kim, J.E. Impact of water consumption on renal function in the general population: A cross-sectional analysis of KNHANES data (2008–2017). Clin. Exp. Nephrol. 2021, 25, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Daimee, U.A.; Wang, M.; Papernov, A.; Sherazi, S.; McNitt, S.; Vidula, H.; Chen, L.; Alexis, J.D.; Kutyifa, V. Renal Function Changes Following Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2017, 120, 2213–2220. [Google Scholar] [CrossRef]
- Schroten, N.F.; Damman, K.; Valente, M.A.; Smilde, T.D.; van Veldhuisen, D.J.; Navis, G.; Gaillard, C.A.; Voors, A.A.; Hillege, H.L. Long-term changes in renal function and perfusion in heart failure patients with reduced ejection fraction. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2016, 105, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Santos, E.; Gowdak, L.H.; Gaiotto, F.A.; Puig, L.B.; Hajjar, L.A.; Zeferino, S.P.; Drager, L.F.; Shimizu, M.H.; Bortolotto, L.A.; De Lima, J.J. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann. Thorac. Surg. 2014, 97, 1617–1623. [Google Scholar] [CrossRef]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef]
- Phruetthiphat, O.A.; Otero, J.E.; Zampogna, B.; Vasta, S.; Gao, Y.; Callaghan, J.J. Predictors for readmission following primary total hip and total knee arthroplasty. J. Orthop. Surg. 2020, 28. [Google Scholar] [CrossRef]

| Preoperative Characteristics | |
|---|---|
| Age, mean ± SD (years) | 66.5 ± 11.1 |
| Sex, n (female/male) | 2109/1645 |
| Body Mass Index, mean ± SD (kg/m2) | 29.5 ± 5.8 |
| ASA score, median (IQR) | 2 (2–3) |
| Medical history, n (%) | |
| Cardiovascular disease | 834 (22.2) |
| Pulmonary disease | 649 (17.3) |
| Renal disease | 246 (6.6) |
| Diabetes | 534 (14.2) |
| High blood pressure | 2399 (63.9) |
| Hypothyreosis | 660 (17.6) |
| Smoker | 450 (12) |
| Depression | 179 (4.8) |
| Rheumatic disease | 70 (1.9) |
| Thrombophilia | 235 (6.3) |
| Haemophilia | 20 (0.5) |
| Long-term aspirin | 672 (17.9) |
| Long-term anticoagulation | 358 (9.5) |
| Blood parameters | |
| Haemoglobin, ±SD (g/dL) | 14.1 ± 1.4 |
| Haematokrit, ±SD (%) | 42.4 ± 3.9 |
| Thrombocytes, ±SD (103/µL) | 242.9 ± 61.8 |
| Serum creatinine, ±SD (µmol/L) | 77.8 ± 27.7 |
| eGFR, ±SD (mL/min) | 84.5 ± 21.2 |
| C-reactive protein (mg/dL) | 0.6 ± 0.9 |
| PTT (s) | 34.2 ± 4.6 |
| Quick (%) | 100.4 ± 13.0 |
| Intraoperative characteristics | |
| Type of surgery, n (hip arthroplasty/knee arthroplasty) | 1948/1806 |
| Use of i.v. tranexamic acid, n (%) | 1013 (27.0) |
| Use of local tranexamic acid, n (%) | 461 (12.3) |
| Type of anaesthesia (general/neuraxial) | 2247/1507 |
| Duration of surgery, mean ± SD (min) | 86.6 ± 23.2 |
| Autologous RBC transfusion, n (%) | 625 (16.6) |
| Postoperative characteristics | |
| Allogeneic RBC transfusion, n (%) | 179 (4.8) |
| Perioperative complications, n (%) | 239 (6.4) |
| Wound site complications (SSI/PJI) | 20 (0.5) |
| Periprosthetic fracture | 6 (0.2) |
| Prosthesis malposition/dislocation | 5 (0.1) |
| Need for Intensive Care | 17 (0.5) |
| Postoperative delirium | 30 (0.8) |
| Decubitus | 20 (0.5) |
| Cardiac complications | 52 (1.4) |
| Pneumonia | 10 (0.3) |
| Fall | 17 (0.5) |
| Hepatic complication | 5 (0.1) |
| Neurological complications | 22 (0.5) |
| Renal complications | 55 (1.5) |
| Gastrointestinal complications | 27 (0.7) |
| DVT/LE | 3 (0.1) |
| Preoperative Variables | B | S.E. | Exp (B) | p |
|---|---|---|---|---|
| (a) | ||||
| Constant | 8.182 | 1.484 | 3576.322 | <0.001 |
| Haemoglobin | −0.779 | 0.069 | 0.459 | <0.001 |
| Cardiovascular disease | 0.897 | 0.192 | 2.453 | <0.001 |
| Body Mass Index | −0.068 | 0.018 | 0.934 | <0.001 |
| Diabetes | −0.941 | 0.282 | 0.390 | 0.001 |
| ASA score | 0.581 | 0.181 | 1.788 | 0.001 |
| Haemophilia | 2.088 | 0.690 | 8.067 | 0.002 |
| Thrombocytes | −0.004 | 0.001 | 0.996 | 0.003 |
| eGFR | −0.012 | 0.004 | 0.988 | 0.004 |
| Age | 0.021 | 0.009 | 1.021 | 0.027 |
| Nagelkerke R Square | 0.320 | |||
| (b) | ||||
| Constant | −4.652 | 0.949 | 0.010 | <0.001 |
| ASA score | 0.545 | 0.131 | 1.724 | <0.001 |
| Age | 0.044 | 0.008 | 1.045 | <0.001 |
| eGFR | −0.012 | 0.003 | 0.988 | 0.001 |
| Quick | −0.015 | 0.005 | 0.985 | 0.002 |
| Nagelkerke R Square | 0.096 |
| Preop. eGFR < 60 mL/min n = 416 (11.1%) | OR (95%CI) | Preop. Hb < 13 g/dl n = 666 (17.7%) | OR (95% CI) | Preop. BMI < 22 kg/m2 n = 198 (5.3%) | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Outcome Measures | ||||||
| RBC transfusion, n (%) | 62 (14.9) | 4.82 (3.48–6.68) | 197 (16.1) | 8.02 (5.87–10.96) | 19 (9.6) | 2.51 (1.39–4.56) |
| Complications, n (%) | 57 (13.7) | 2.75 (2.00–3.78) | ||||
| Wound site complications | 2 (0.5) | 0.89 (0.21–3.85) | ||||
| Periprosthetic fracture | 2 (0.5) | 4.02 (0.74–22.04) | ||||
| Prosthesis malposition/migration | 1 (0.2) | 2.01 (0.22–18.0) | ||||
| Need for Intensive Care | 4 (1.0) | 2.48 (0.81–7.65) | ||||
| Postoperative delirium | 4 (1.0) | 1.24 (0.43–3.56) | ||||
| Decubitus | 1 (0.2) | 0.42 (0.06–3.15) | ||||
| Cardiac complications | 12 (2.9) | 2.45 (1.27–4.7) | ||||
| Pneumonia | 1 (0.2) | 0.89 (0.11–7.05) | ||||
| Fall | 5 (1.2) | 3.37 (1.18–9.61) | ||||
| Hepatic complication | 1 (0.2) | 2.0 (0.22–18.0) | ||||
| Neurological complications | 3 (0.7) | 1.27 (0.37–4.3) | ||||
| Renal complications | 28 (6.7) | 8.84 (5.16–15.16) | ||||
| Gastrointestinal complications | 6 (1.4) | 2.31 (0.93–5.76) | ||||
| DVT/LE | 1 (0.2) | 4.02 (0.36–44.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakuscheit, A.; Schaefer, N.; Roedig, J.; Luedemann, M.; von Hertzberg-Boelch, S.P.; Weissenberger, M.; Schmidt, K.; Holzapfel, B.M.; Rudert, M. Modifiable Individual Risks of Perioperative Blood Transfusions and Acute Postoperative Complications in Total Hip and Knee Arthroplasty. J. Pers. Med. 2021, 11, 1223. https://doi.org/10.3390/jpm11111223
Jakuscheit A, Schaefer N, Roedig J, Luedemann M, von Hertzberg-Boelch SP, Weissenberger M, Schmidt K, Holzapfel BM, Rudert M. Modifiable Individual Risks of Perioperative Blood Transfusions and Acute Postoperative Complications in Total Hip and Knee Arthroplasty. Journal of Personalized Medicine. 2021; 11(11):1223. https://doi.org/10.3390/jpm11111223
Chicago/Turabian StyleJakuscheit, Axel, Nina Schaefer, Johannes Roedig, Martin Luedemann, Sebastian Philipp von Hertzberg-Boelch, Manuel Weissenberger, Karsten Schmidt, Boris Michael Holzapfel, and Maximilian Rudert. 2021. "Modifiable Individual Risks of Perioperative Blood Transfusions and Acute Postoperative Complications in Total Hip and Knee Arthroplasty" Journal of Personalized Medicine 11, no. 11: 1223. https://doi.org/10.3390/jpm11111223