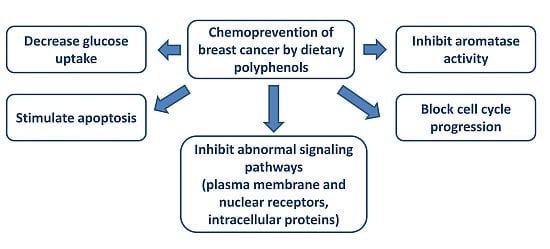
Chemoprevention of Breast Cancer by Dietary Polyphenols
Abstract
:1. Introduction
2. Breast Cancer—General Aspects
3. Polyphenols—General Aspects
4. In Vitro Effect of Polyphenols
4.1. Antioxidant Activity of Polyphenols
| Class of Flavonoid | Chemical Structure | Representative Members | Dietary Sources |
|---|---|---|---|
| Flavonoids C6–C3–C6 |  | ||
| Flavonols |  | Quercetin Kaempferol Myricetin | Onions (Allium cepa), spinach (Spinacia oleracea), cauliflower (Brassica oleracea Botrytis Group), strawberries (Fragaria ananassa) |
| Flavones |  | Apigenin Luteolin | Celery (Apium graveolens), parsley (Petroselinum crispum), artichoke (Cynara scolymus) |
| Flavan-3-ols/proto-anthocyanidins |  | Epicatechin Epigallocatechin Epigallocatechin-3-O-gallate (EGCG) Procyanidin 2 | Apricots (Prunus armeniaca), sour cherries (Prunus cerasus), grapes (Vitis ssp.), blackberries (Rubus ssp.), apples (Malus domestica) dark chocolate—seeds of cocoa (Theobroma cacao), mint (Mentha rodundifolia), basil (Ocimum basilicum), rosemary (Rosemarinus officinalis), sage (Salvia officinalis), dill (Anetheum graveolens), green tea (Camellia sinensis), hazelnuts (Corylus avellana), pecans (Carya illinoensis), almonds (Prunus dulcis), pistachios (Pistachio vera), walnuts (Juglans ssp.) |
| Anthocyanidins/Anthocynins |  | Cyanidin Pelargonidin Delphinidin Malvidin | Red grapes (Vitis labrusca, Vitis vinifera), cranberry (Vaccinium macrocarpon), blackberry (Rubus ssp.), elderberry (Sambucus nigra), blueberry (Vacciunium corymbosum), blackcurrant (Ribes nigrum), sweet cherries (Prunus avium), sour cherries (Prunus cerasus), plums (Prunus domestica), peaches (Prunus persica) |
| Flavanones |  | Naringerin Hesperitin | Orange (Citrus sinensis), lemon (Citrus lemon), mandarin (Citrus reticulate), grapefruit (Citrus paradisi), tomatoes (Solamun lycopersicum) |
| Isoflavones |  | Genistein Daidzein Glycitin | Soybean (Glycine max), beans (Phaseolus vulgaris), green peas (Pisum sativum) |
| Class of Non-Flavonoids | Chemical Structure | Representative Members | Dietary Sources |
|---|---|---|---|
| Phenolic acids—Benzoic acids/hydroxybenzoates C6–C1 |  | Gallic acid p-hydroxy-benzoic Syringic acid Vanillic acid | Clove buds (Eugenia caryophyllata) Grains: wheat (Triticum vulgare), rice (Oryza sativa), oat (Avena sativa), rye (Secale cereale), barley (Hordeum vulgare) Dates (Phoenix dactylifera) |
| Phenolic acids—Cinnamic acids/hydroxycinnamates C6–C3 |  | p-coumaric acid Caffeic acid Ferulic acid Chlorogenic acid | Apples (Malus domestica) Dates (Phoenix dactylifera) Green coffee beans (Coffea arabica) Carrots (Daucus carota) |
| Stilbenes C6–C2–C6 |  | Resveratrol | Red wine, peanuts (Arachis hypogaea), red cabbage (Brassica olearaceae Capitata Group), spinach (Spinacia oleracea) |
| Other polyphenols |  | Curcumin (a) Rosmarinic acid (b) Gingerol (c) | Turmeric (Curcuma longa) Rosemary (Rosmarinus officinalis) Ginger (Zingiber officinale) |
4.2. Polyphenols and Aromatase Inhibitor Activity

4.3. Reversal of Glycolytic Metabolism by Polyphenols

4.4. Regulation of Cell Cycle and Apoptosis by Polyphenols


4.5. Estrogen Receptors and Polyphenols

4.6. Effect of Polyphenols on Plasma Membrane Receptors and on Signaling Pathways
4.7. Epigenetic Mechanisms and Polyphenols
4.8. Breast Cancer Stem Cells (BCSC) and Polyphenols
4.9. EMT and Polyphenols
4.10. Administration of Polyphenols as Nanoparticles
4.11. Combined Applications of Polyphenols in Vitro
5. In Vivo Experiments
5.1. The Effect of Polyphenols on Tumor Growth—Animal Models
| Author, Year | Animals | Dose and Duration of Administration | Result |
|---|---|---|---|
| Genistein | |||
| Murrill W.B. et al., 1996 [216] | Pre-pubertal Sprague-Dawley rats with DMBA induced carcinoma | 500 μg/g body weight in P16, P18, P20 | Reduction in carcinoma incidence |
| Jin Z. et al., 2002 [217] | Pre-pubertal rats with DMBA induced carcinoma | 500 mg/kg body weight in P7, P20 | Reduction in tumor multiplicity by 60% |
| Cabanes A. et al., 2004 [221] | Pre-pubertal female rats with DMBA induced carcinoma | 50 µg (injection) daily from P7 to P20 | Reduction in the size of the mammary epithelial area, reduction in number of TEB, increased density of lobulo-alveolar structures (increased differentiation), up-regulation of breast cancer tumor suppressor gene 1 (BRCA1) mRNA |
| Ju Y.H. et al., 2001 [218] | Ovariectomized athymic mice with MCF-7 xenografts | 125, 1000 μg/g body weight in the diet for 22 weeks | Tumor size was increased in dose-dependent manner; cell proliferation was enhanced at concentration >250 μg/g; increased in pS2, an estrogen responsive gene at concentration >500 μg/g |
| Ju Y.H. et al., 2002 [219] | Ovariectomized athymic BALB/c (nude) mice with MCF-7 xenografts | 1000 ppm (1000 μg/g body weight) | Genistein canceled the inhibitory effect of tamoxifen, decreased estradiol level in plasma, increased expression estradiol regulated genes (pS2, progesterone, cyclin D1) |
| Jin Z. et al., 2002 [217] | Transgenic mice for MMTV-neu gene | 250 mg/kg for 7 weeks | Mammary tumor latency delayed compared to controls; no reduction in in the number or tumor size |
| Kijkuokool P. et al., 2006 [220] | Adult female Sprague-Dawley rats exposed to NMU | 1 mg/kg body weight daily subcutaneous injection for 20 weeks | Increased tumor cross-sectional area, increased tumor multiplicity, but not tumor incidence |
| Daidzein | |||
| Constantinou A.I. et al., 2001 [222] | Female Sprague-Dawley rats with DMBA breast carcinoma induction | 200 mg/kg diet | Tumor incidence and survival similar to control groups; reduction in tumor multiplicity by 32%; increased median tumor latency |
| Jin Z. et al., 2002 [217] | Transgenic mice for MMTV-neu gene | 250 mg/kg for 7 weeks | Mammary tumor latency delayed compared to controls; no reduction in in the number or tumor size |
| Lamartiniere C.A. et al., 2002 [223] | Virgin female rats | 250 and 1000 mg/kg in the diet, 2 weeks prior to breeding till 50 day postpartum | Moderate reduction in ovarian and uterine weights and mammary gland size; reduced body weight; reduction in circulating progesterone |
| Ju Y.H. et al., 2006 [224] | Ovariectomized athymic mice with MCF-7 human xenografts | 125 to 1000 ppm (125 to 1000 μg/g body weight) | No statistical significant reduction in tumor size and proliferation |
| Resveratrol | |||
| Banerjee S. et al., 2002 [225] | Female Sprague-Dawley rats with DMBA breast carcinoma induction | 10 ppm | Reduction in the incidence (by 45%) and multiplicity (by 55%) of the tumors; increased latency period; suppressed COX-2, MMP-9, NF-kB; no effect on body weight or tumor volume |
| Whitsett T. et al., 2006 [226] | Female Sprague-Dawley rats with DMBA breast carcinoma induction | 1 g/kg in the diet | Suppression of mammary carcionogenesis: less number of tumors per rat, longer tumor latency; reduced proliferation; increased apoptosis in epithelial cells of TEB; reduced toxicity: no alterations in body weight |
| Singh B. et al., 2014 [129] | August Copenhagen Irish rats (rodent model of breast carcionogenesis) | 50 mg subcutaneous pellet per month, 8 months | Decreased tumor incidence and increases latency in mammary tumors induced by estradiol; upregulated NRF2, a regulator of the anti-oxidant response; induced apoptosis (increased p53 and PARP cleavage) in mammary tissue |
| EGCG | |||
| Whitsett T. et al., 2006 [226] | Female Sprague Dawley rats with DMBA and NMU mammary cancer induction | 0.065% in the drinking water | Not efficient in reduction of breast cancer incidence at these doses |
| Quercetin | |||
| Verma A.K. et al., 1998 [227] | Female Sprague Dawley rats with DMBA and NMU mammary cancer induction | 5% in the diet | Reduction in the number of tumors; decreased tumor multiplicity; no detectable signs of toxicity (similar body weight in treated and control rats) |
| Singh B. et al., 2010 [228] | Female August Copenhagen Irish (ACI) rats | 2.5 g/kg in diet, 8 months | No induction of tumors in ACI rats; did not protect against estrogen-induced tumors; did not confer protection against breast cancer and may worsen breast cancer status regularly exposed to estradiol |
| Curcumin | |||
| Masuelli L. et al., 2013 [121] | BALB-neuT transgenic mice for neu oncogene | n.m. | Increased tumor-free survival; reduction in tumor multiplicity; safe to be administrated: no modification in hematological and clinical chemistry parameters |
5.2. Involvement of Polyphenols in Modulation of Metastasis and Angiogenesis
5.3. Combined Administration of Polyphenols–In Vivo Studies
6. Clinical Implications of Polyphenols
6.1. Bioavailability of Polyphenols in Human Body
| Source/Polyphenol | Dose | Concentration in Plasma (µM) | Half-Life (h) | Ref. |
|---|---|---|---|---|
| Onions | 100 mg quercetin eq | 7.6 | 10.9 | [256] |
| Apples | 107 mg quercetin eq | 0.3 | 23.0 | [257] |
| Quercetin | 50 mg | 0.29 | 15.0 | [258] |
| Orange juice | 126 mg hesperitin eq | 2.2 | 2.2 | [259] |
| Orange juice | 23 mg narigerin eq | 0.64 | 1.3 | [259] |
| Grapefruit juice | 199 mg narigerin eq | 5.99 | 2.2 | [259] |
| Chocolate (80 g) | 164 mg epicatechin eq | 0.7 | 1.9–2.3 | [260] |
| Red wine (120 mL) | 35 mg catechin eq | 0.091 | 3.1 | [261] |
| EGCG | 800 mg | 2.33 | 1.9–4.6 | [262] |
| Soy beverage | 0.6 mg/kg daidzein eq | 0.3 | 3.4 | [263] |
| Soy beverage | 1 mg/kg genistein eq | 0.65 | 7.9 | [263] |
| Daidzein | 50 mg | 0.76 | 9.3 | [264] |
| Genistein | 50 mg | 1.26 | 6.8 | [264] |
6.2. Chemoprevention of Breast Cancer by Polyphenols in Humans
| Author, Year | Date of Study | Cases 1 | Ctrl 1 | Diet | Dose 2 | OR/HR/RR (95% CI) | Conclusion |
|---|---|---|---|---|---|---|---|
| Liu X.O. et al., 2014 [270] | 1990–2013 | 9299 | 11,412 | Soy (soy protein, soy food, soya- bean milk) | 1–8 times/week 12.9–500 mg/day 3 | 0.65 (0.43–0.99) | Soy intake was associate with reduction in breast cancer risk (Chinese women) |
| Nagata C. et al., 2014 a [267] | 1985–2005 | 2531 | 25,332 | Soy (tofu, soybeans, miso soup) | 1–3 times/week | 0.62 (0.38–1.01) to 1.59 (0.90–2.81) | Soy intake was associated with moderate and strong reduction of breast cancer in post-menopausal Japanese women |
| Fritz H. et al., 2013 b [269] | 1992–2012 | 1830 c | n.m. | Soy (soy food, soy protein, genistein, IF) | Soy 13.03–65.7 g/day IF 7.48–62.68 mg/day | 0.25 (0.10–0.61) to 1.19 (0.76–1.85) | Soy intake was associated with no change and increase survival, no change and decrease recurrence of breast cancer in Chinese, Korean, USA, Shanghai women, respectively |
| Guha N. et al., 2009 [275] | 1997–2000 | 1954 d | n.m. | Daidzein, genistein, glycetin | Daidzein 1.5–9.6 mg/day 0.1–7.8 µg/day Genistein 2.2–13 mg/day 0.1–7 µg/day Glycetin 8.2–15 µg/day 3.6–8.2 µg/day | Daidzein 0.71 (0.45–1.11) to 1.16 (0.81–1.68) Genistein 0.72 (0.46–1.13) to 1.09 (0.76–1.58) Glycetin 0.68 (0.46–1.01) to 1.01 (0.71–1.43) | Decreased risk of breast cancer recurrence was associated with high daidzein and glycetin intake in postM women Women treated with Tamoxifen presented 60% decrease in breast cancer recurrence after daidzein intake |
| Trock B.J. et al., 2006 a [215] | 1978–2004 | 7453 | 16,521 | Soy protein and tofu | 1–5 times/week 1.6–3.5 g/day | 0.86 (0.75–0.99) preM 0.70 (0.58–0.85) postM 0.77 (0.60–0.98) | Increased soy intake was associated with modest reduction in breast cancer risk Inverse association between soy exposure and breast cancer risk in preM (“stronger”) and postM women Caution with interpreting the data due to high heterogeneity of soy exposure |
| Wu A.H. et al., 2002 [276] | 1995–1998 | 501 | 594 | Tofu—adolescence IF—adult | 1–3 times/month +4 times/week >1.79–6.24 mg/1000 kcal >12.68 mg/1000 kcal | 0.75 (0.48–1.15) 0.51 (0.31–0.84) 0.76 (0.53–1.09) 0.51 (0.33–0.78) | High soy intake during adolescence and adult life was associated with reduced risk of breast cancer (Chinese, Japanese, Filipino women in Los Angeles) |
| Shu X.O. et al., 2001 [265] | 1996–1998 | 296 | 359 | Soy food—13–15 years, adolescence | 5.4 g/day | 0.51 (0.40–0.65) | Adolescent soy food intake was inversely associated with breast cancer risk |
7. Conclusions and Further Progress
| Pros | Cons |
|---|---|
|
|
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Crowell, J.A.; Steele, V.E.; Lubet, R.A.; Malone, W.A.; Boone, C.W.; Kopelovich, L.; Hawk, E.T.; Lieberman, R.; Lawrence, J.A.; et al. Progress in cancer chemoprevention: Development of diet-derived chemopreventive agents. J. Nutr. 2000, 130 (2S Suppl.), 467S–471S. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.; Newton, D. Chemoprevention of cancer with retinoids. Fed. Proc. 1979, 38, 2528–2534. [Google Scholar] [PubMed]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Izzotti, A.; D’Agostini, F.; Balansky, R.M.; Noonan, D.; Albini, A. Multiple points of intervention in the prevention of cancer and other mutation-related diseases. Mutat. Res. 2001, 480–481, 9–22. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular signaling perturbation by natural products. Cell. Signal. 2009, 21, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Agoston, V.; Csermely, P.; Pongor, S. Multiple weak hits confuse complex systems: A transcriptional regulatory network as an example. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, (5 Pt 1). 051909. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P. Strong links are important, but weak links stabilize them. Trends Biochem. Sci. 2004, 29, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and breast cancer: Focus on angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T. The mammary gland and its origin during synapsid evolution. J. Mammary Gland Biol. Neoplasia 2002, 7, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.S.; Werb, Z. Stromal effects on mammary gland development and breast cancer. Science 2002, 296, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.I.; Aumsuwan, P.; Khan, I.A.; Walker, L.A.; Dasmahapatra, A.K. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem. Res. Toxicol. 2012, 25, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, S.J. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod. Pathol. 2010, 23 (Suppl. S2), S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, M.J.; Opdahl, S.; Hagen, A.I.; Romundstad, P.R.; Akslen, L.A.; Haugen, O.A.; Vatten, L.J.; Bofin, A.M. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res. Treat. 2013, 140, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staaf, J.; Ringner, M. Making breast cancer molecular subtypes robust? J. Natl. Cancer Inst. 2015, 107, 386. [Google Scholar] [CrossRef] [PubMed]
- Jonat, W.; Pritchard, K.I.; Sainsbury, R.; Klijn, J.G. Trends in endocrine therapy and chemotherapy for early breast cancer: A focus on the premenopausal patient. J. Cancer Res. Clin. Oncol. 2006, 132, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Baselga, J. Management of breast cancer with targeted agents: Importance of heterogeneity. [corrected]. Nat. Rev. Clin. Oncol. 2010, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Conte, P. Metastatic breast cancer: Therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist 2009, 14, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Rice, J. Metastasis: The rude awakening. Nature 2012, 485, S55–S57. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Donehower, R.C. The clinical pharmacology of paclitaxel (Taxol). Semin. Oncol. 1993, 20 (Suppl. S3), 16–25. [Google Scholar] [PubMed]
- Arbuck, S.G.; Strauss, H.; Rowinsky, E.; Christian, M.; Suffness, M.; Adams, J.; Oakes, M.; McGuire, W.; Reed, E.; Gibbs, H.; et al. A reassessment of cardiac toxicity associated with Taxol. J. Natl. Cancer Inst. Monogr. 1993, 15, 117–130. [Google Scholar] [PubMed]
- Ahmad, A. Pathways to breast cancer recurrence. ISRN Oncol. 2013, 2013, 290568. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int J Mol. Sci 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Jaganath, I.; Clifforrd, M. Plant Secondary Metabolites, 1st ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 1–25. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R. Phenolic Compounds Biochemistry, 1st ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–34. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Van Sumere, C. Methods in Plant Biochemistry: Plant Phenolics, 1st ed.; Academic Press: San Diego, CA, USA, 1989; pp. 29–73. [Google Scholar]
- Jaganath, I.; Crozier, A.; Poquet, L.; Clifford, M.; Williamson, G. Plant Phenolics and Human Health; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–89. [Google Scholar]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Yordi, E.; Perez, E.; Matos, M.; Villares, E. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence, 1st ed.; InTech: Rijeka, Croatia or Shanghai, China, 2012; pp. 23–48. [Google Scholar]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Sandoval-Acuna, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Garcia, C.R.; Pinzon, J.R.; Chaur, M.N.; Brumaghim, J.L. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J. Inorg. Biochem. 2011, 105, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ramawat, K.; Merillon, J. Natural Products, Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes, 1st ed.; Springer: Heidelberg, Germany, 2013; pp. 1541–2662. [Google Scholar]
- Marais, J.; Deavours, B.; Dixon, R.; Ferreira, D. The Science of Flavonoids, 1st ed.; Springer: Columbus, OH, USA, 2006; pp. 1–46. [Google Scholar]
- Pluchino, L.A.; Wang, H.C. Chronic exposure to combined carcinogens enhances breast cell carcinogenesis with mesenchymal and stem-like cell properties. PLoS ONE 2014, 9, e108698. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Neagoe, I.B. The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [Google Scholar] [PubMed]
- Singh, N.; Zaidi, D.; Shyam, H.; Sharma, R.; Balapure, A.K. Polyphenols sensitization potentiates susceptibility of MCF-7 and MDA MB-231 cells to Centchroman. PLoS ONE 2012, 7, e37736. [Google Scholar] [CrossRef] [PubMed]
- Akbas, S.H.; Timur, M.; Ozben, T. The effect of quercetin on topotecan cytotoxicity in MCF-7 and MDA-MB 231 human breast cancer cells. J. Surg. Res. 2005, 125, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Koshy, L.; Dwarakanath, B.S.; Raj, H.G.; Chandra, R.; Mathew, T.L. Suicidal oxidative stress induced by certain antioxidants. Indian J. Exp. Biol. 2003, 41, 1273–1278. [Google Scholar] [PubMed]
- Nadal-Serrano, M.; Pons, D.G.; Sastre-Serra, J.; Blanquer-Rossello Mdel, M.; Roca, P.; Oliver, J. Genistein modulates oxidative stress in breast cancer cell lines according to ERalpha/ERbeta ratio: Effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. Int. J. Biochem. Cell Biol. 2013, 45, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Ahamed, M.; Ahmad, J.; Majeed Khan, M.A.; Musarrat, J.; Al-Khedhairy, A.A.; Alrokayan, S.A. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem. Toxicol. 2012, 50, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Brueggemeier, R.W.; Hackett, J.C.; Diaz-Cruz, E.S. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005, 26, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M.; Gustafsson, J.A. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Kellis, J.T., Jr.; Vickery, L.E. Inhibition of human estrogen synthetase (aromatase) by flavones. Science 1984, 225, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Shin, Y.G.; Kim, I.H.; Pezzuto, J.M. Inhibition of aromatase activity by flavonoids. Arch. Pharm. Res. 1999, 22, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Bankemper, T.; Kidney, R.; Do, T.; Onate, A.; Thowfeik, F.S.; Merino, E.J.; Paula, S.; Ma, L. Investigation of fluorinated and bifunctionalized 3-phenylchroman-4-one (isoflavanone) aromatase inhibitors. Bioorg. Med. Chem. 2014, 22, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hodek, P.; Trefil, P.; Stiborova, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
- Li, F.; Wong, T.Y.; Lin, S.M.; Chow, S.; Cheung, W.H.; Chan, F.L.; Chen, S.; Leung, L.K. Coadministrating luteolin minimizes the side effects of the aromatase inhibitor letrozole. J. Pharmacol. Exp. Ther. 2014, 351, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, L.; Lin, S.M.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Z.; Lao, K.J.; Hu, J.; Pang, T.; Jiang, Z.Z.; Yuan, H.L.; Miao, J.S.; Chen, X.; Ning, S.S.; Xiang, H.; et al. Discovery of novel aromatase inhibitors using a homogeneous time-resolved fluorescence assay. Acta Pharmacol. Sin. 2014, 35, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, K.; Amato, E.; Bankemper, T.; Agard, H.; Steller, J.; Keeler, J.M.; Roy, D.; McCallum, A.; Paula, S.; Ma, L. Development of a new class of aromatase inhibitors: Design, synthesis and inhibitory activity of 3-phenylchroman-4-one (isoflavanone) derivatives. Bioorg. Med. Chem. 2012, 20, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Lee, H.H.; Kao, M.C.; Lin, J.K. Black tea polyphenol theaflavins inhibit aromatase activity and attenuate tamoxifen resistance in HER2/neu-transfected human breast cancer cells through tyrosine kinase suppression. Eur. J. Cancer 2004, 40, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Sakamoto, Y.; Ogata, A.; Nagai, F.; Mikuriya, H.; Numazawa, M.; Yamada, K.; Aoki, N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002, 40, 925–933. [Google Scholar] [CrossRef]
- Van Duursen, M.B.; Nijmeijer, S.M.; de Morree, E.S.; de Jong, P.C.; van den Berg, M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 2011, 289, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J. Prevention of Cancer through Lifestyle Changes. Evid. Based Complement. Altern. Med. 2004, 1, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Keijer, J.; Bekkenkamp-Grovenstein, M.; Venema, D.; Dommels, Y.E. Bioactive food components, cancer cell growth limitation and reversal of glycolytic metabolism. Biochim. Biophys. Acta 2011, 1807, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, I.M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012, 209, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C. Cancer Cell Metabolism, Epigenetics and the Potential Influence of Dietary Components—A Perspective. Biomed. Res. 2012, 23, 1–21. [Google Scholar]
- Wong, N.; de Melo, J.; Tang, D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int. J. Cell Biol. 2013, 2013, 242513. [Google Scholar] [CrossRef] [PubMed]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.H.; Lee, K.H. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1alpha activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Araujo, I.; Costa, T.; Correia-Branco, A.; Faria, A.; Martel, F.; Keating, E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp. Cell Res. 2013, 319, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677–25695. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Correia-Branco, A.; Araujo, J.R.; Guimaraes, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.S.; Zancan, P.; Marcondes, M.C.; Ramos-Santos, L.; Meyer-Fernandes, J.R.; Sola-Penna, M.; da Silva, D. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 2013, 95, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.; van Opstal, A.; Boonstra, J. Focal adhesion signaling and actin stress fibers are dispensable for progression through the ongoing cell cycle. J. Cell Sci. 2007, 120, (Pt 1). 66–76. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A. Cell cycle: Regulating chromosome segregation. Nat. Rev. Mol. Cell Biol. 2014, 15, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK inhibitors: Cell cycle regulators and beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- D’Andrilli, G.; Kumar, C.; Scambia, G.; Giordano, A. Cell cycle genes in ovarian cancer: Steps toward earlier diagnosis and novel therapies. Clin. Cancer Res. 2004, 10, 8132–8141. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Mjelle, R.; Hegre, S.A.; Aas, P.A.; Slupphaug, G.; Drablos, F.; Saetrom, P.; Krokan, H.E. Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair 2015, 30, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, S. Targeting apoptosis: Selected anticancer strategies. Nat. Rev. Drug Discov. 2008, 7, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.V.; Paczulla, A.M.; Klonisch, T.; Dimgba, F.N.; Rao, S.B.; Roberg, K.; Schweizer, F.; Lengerke, C.; Davoodpour, P.; Palicharla, V.R.; et al. Interconnections between apoptotic, autophagic and necrotic pathways: Implications for cancer therapy development. J. Cell. Mol. Med. 2013, 17, 12–29. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.; Lai, A.; Carroll, J.S.; Sergio, C.M.; Mitchell, C.J.; Sarcevic, B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol. Cell. Endocrinol. 2005, 229, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, F.; Lubet, R.A.; de Luca, A.; Grubbs, C.; Ericson, M.E.; D’Alessio, A.; Normanno, N.; Dong, Z.; Bode, A.M. Quercetin-3-methyl ether inhibits lapatinib-sensitive and -resistant breast cancer cell growth by inducing G(2)/M arrest and apoptosis. Mol. Carcinog. 2013, 52, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Bae, S.M.; Ahn, W.S. Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Arch. Pharm. Res. 2008, 31, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wong, C.; John Bennett, D.; Wu, J.M. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: An in silico and biochemical approach targeting integrin alphavbeta3. Int. J. Cancer 2011, 129, 2732–2743. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Hochegger, H.; Takeda, S.; Hunt, T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat. Rev. Mol. Cell Biol. 2008, 9, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Singh, P.; Panda, D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010, 277, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, Y.; Wang, A.; Wang, R.H.; Wang, X.; Cao, L.; Deng, C.X. Genistein inhibits Brca1 mutant tumor growth through activation of DNA damage checkpoints, cell cycle arrest, and mitotic catastrophe. Cell Death Differ. 2007, 14, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Blanquer-Rossello, M.M.; Sastre-Serra, J.; Oliver, J.; Roca, P. Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. J. Cell. Biochem. 2014, 115, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2009, 20, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Jin, H.; Yang, F.; Zhu, H.; Cai, J. Apigenin induced MCF-7 cell apoptosis-associated reactive oxygen species. Scanning 2014, 36, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, L.; Cappelletti, V.; Miodini, P.; Ronchi, E.; Brivio, M.; di Fronzo, G. Genistein in the control of breast cancer cell growth: Insights into the mechanism of action in vitro. Cancer Lett. 1998, 130, 143–152. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Passi, N.; Maheshwari, R.K. Green tea polyphenol and epigallocatechin gallate induce apoptosis and inhibit invasion in human breast cancer cells. Cancer Biol. Ther. 2007, 6, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, E.P.; Almeida, G.M.; Jones, G.D.; Manson, M.M. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol. Cancer Ther. 2007, 6, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Horiguchi, H.; Oguma, E.; Kayama, F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J. Nutr. Biochem. 2010, 21, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [PubMed]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Jang, B.H.; Go, H.; Shin, Y.C.; Ko, S.G. Apigenin induces caspase-dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2-overexpressing SKBR3 breast cancer cells. Mol. Med Rep. 2015, 12, 2977–2984. [Google Scholar] [PubMed]
- Masuelli, L.; Benvenuto, M.; Fantini, M.; Marzocchella, L.; Sacchetti, P.; di Stefano, E.; Tresoldi, I.; Izzi, V.; Bernardini, R.; Palumbo, C.; et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J. Biol. Regul. Homeost. Agents 2013, 27, 105–119. [Google Scholar] [PubMed]
- Chen, Z.P.; Schell, J.B.; Ho, C.T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179. [Google Scholar] [CrossRef]
- Eddy, S.F.; Kane, S.E.; Sonenshein, G.E. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007, 67, 9018–9023. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Viacava, P.; Naccarato, A.G.; Collecchi, P.; Menard, S.; Castronovo, V.; Bevilacqua, G. The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J. Pathol. 1997, 182, 36–44. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.M.; Ganea, C.; Georgescu, L.; Varadi, T.; Shrestha, D.; Baran, I.; Katona, E.; Nagy, P.; Szollosi, J. Epigallocatechin 3-O-gallate induces 67 kDa laminin receptor-mediated cell death accompanied by downregulation of ErbB proteins and altered lipid raft clustering in mammary and epidermoid carcinoma cells. J. Nat. Prod. 2014, 77, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J. Natl. Cancer Inst. 1998, 90, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Dodin, S.; Blanchet, C.; Marc, I. [Phytoestrogens in menopausal women: A review of recent findings]. Med. Sci. 2003, 19, 1030–1037. [Google Scholar]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Perspect. 2008, 116, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef] [PubMed]
- This, P.; de Cremoux, P.; Leclercq, G.; Jacquot, Y. A critical view of the effects of phytoestrogens on hot flashes and breast cancer risk. Maturitas 2011, 70, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Arnould, S.; Scalbert, A.; Manach, C. Isoflavones and the prevention of breast and prostate cancer: New perspectives opened by nutrigenomics. Br. J. Nutr. 2008, 99 (E Suppl. S1), ES78–ES108. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; Couse, J.F.; Korach, K.S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb. Perspect. Biol. 2010, 2, a001057. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Chung, J. Curcumin inhibition of the functional interaction between integrin alpha6beta4 and the epidermal growth factor receptor. Mol. Cancer Ther. 2011, 10, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, F.; Cai, J.Y.; Yang, P.H.; Liang, Z.H. In-situ detection of resveratrol inhibition effect on epidermal growth factor receptor of living MCF-7 cells by Atomic Force Microscopy. Biosens. Bioelectron. 2014, 56, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pianetti, S.; Guo, S.; Kavanagh, K.T.; Sonenshein, G.E. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002, 62, 652–655. [Google Scholar] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Li, L.Y.; Lee, Y.J. Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J. Cell. Biochem. 2008, 105, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Barnes, S. The role of soy products in reducing risk of cancer. J. Natl. Cancer Inst. 1991, 83, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Liu, X.E.; Huang, D.S. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med. Rep. 2012, 6, 1267–1270. [Google Scholar] [PubMed]
- Long, X.; Fan, M.; Bigsby, R.M.; Nephew, K.P. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol. Cancer Ther. 2008, 7, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef] [PubMed]
- Mahn, K.; Borras, C.; Knock, G.A.; Taylor, P.; Khan, I.Y.; Sugden, D.; Poston, L.; Ward, J.P.; Sharpe, R.M.; Vina, J.; et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005, 19, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, L.; Chalabi, N.; Delort, L.; Bignon, Y.J.; Bernard-Gallon, D.J. Differential expression of genes induced by resveratrol in human breast cancer cell lines. Nutr. Cancer 2006, 56, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Levenson, A.S.; Gehm, B.D.; Pearce, S.T.; Horiguchi, J.; Simons, L.A.; Ward, J.E., 3rd; Jameson, J.L.; Jordan, V.C. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int. J. Cancer 2003, 104, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Holly, J.M.; Perks, C.M. Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front. Endocrinol. (Lausanne) 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chen, Y.; Liu, R.; Zhang, H.; Zhang, Y. Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch. Pharm. Res. 2014, 37, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Pan, M.H.; Chiou, Y.S.; Cheng, A.C.; Huang, H. Resveratrol modulates MED28 (Magicin/EG-1) expression and inhibits epidermal growth factor (EGF)-induced migration in MDA-MB-231 human breast cancer cells. J. Agric. Food Chem. 2011, 59, 11853–11861. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Gao, Q.G.; Wong, M.S. Mechanism involved in genistein activation of insulin-like growth factor 1 receptor expression in human breast cancer cells. Br. J. Nutr. 2007, 98, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wong, M.S. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J. Clin. Endocrinol. Metab. 2004, 89, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [PubMed]
- Bjornstrom, L.; Sjoberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; Schiff, R.; Osborne, C.K. Targeting HER2 for the treatment of breast cancer. Annu. Rev. Med. 2015, 66, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lu, J.; Subramanian, A.; Sonenshein, G.E. Microarray-assisted pathway analysis identifies mitogen-activated protein kinase signaling as a mediator of resistance to the green tea polyphenol epigallocatechin 3-gallate in her-2/neu-overexpressing breast cancer cells. Cancer Res. 2006, 66, 5322–5329. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Miyoshi, N.; Kawabata, K.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β2-adrenergic signaling. Arch. Biochem. Biophys. 2014, 557, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Bouchal, J.; Burkadze, G.; Kolar, Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 2006, 73, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Dolled-Filhart, M.; McCabe, A.; Giltnane, J.; Cregger, M.; Camp, R.L.; Rimm, D.L. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006, 66, 5487–5494. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Xia, W.; Wang, J.C.; Kwong, K.Y.; Spohn, B.; Wen, Y.; Pestell, R.G.; Hung, M.C. β-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. USA 2000, 97, 4262–4266. [Google Scholar] [CrossRef] [PubMed]
- Burkhalter, R.J.; Westfall, S.D.; Liu, Y.; Stack, M.S. Lysophosphatidic Acid Initiates Epithelial to Mesenchymal Transition and Induces β-Catenin-mediated Transcription in Epithelial Ovarian Carcinoma. J. Biol. Chem. 2015, 290, 22143–22154. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.G. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NF-κB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33 (Suppl.), 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Issa, J.P. DNA methylation profiling in cancer. Expert Rev. Mol. Med. 2010, 12, e23. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Laird, P.W. Cancer epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation through repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Veeck, J.; Esteller, M. Breast cancer epigenetics: From DNA methylation to microRNAs. J. Mammary Gland Biol. Neoplasia 2010, 15, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Momparler, R.L. Cancer epigenetics. Oncogene 2003, 22, 6479–6483. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Silva, J.M.; Dominguez, G.; Garcia, J.M.; Cantos, B.; Rodriguez, R.; Larrondo, F.J.; Provencio, M.; Espana, P.; Bonilla, F. Concomitant expression of p16INK4a and p14ARF in primary breast cancer and analysis of inactivation mechanisms. J. Pathol. 2003, 199, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, V.; Stefansson, O.A.; Bodvarsdottir, S.K.; Hilmarsdottir, H.; Jonasson, J.G.; Eyfjord, J.E. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006, 8, R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Guilleret, I.; Benhattar, J. Unusual distribution of DNA methylation within the hTERT CpG island in tissues and cell lines. Biochem. Biophys. Res. Commun. 2004, 325, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Heeg, S.; von Werder, A.; Goessel, G.; Fulda, C.; Doebele, M.; Nakagawa, H.; Beijersbergen, R.; Blum, H.E.; Opitz, O.G. Differential transcriptional regulation of human telomerase in a cellular model representing important genetic alterations in esophageal squamous carcinogenesis. Carcinogenesis 2005, 26, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Berletch, J.B.; Liu, C.; Love, W.K.; Andrews, L.G.; Katiyar, S.K.; Tollefsbol, T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J. Cell. Biochem. 2008, 103, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int. J. Cancer 2009, 125, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med. Oncol. 2010, 27, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K.; Liu, W.; Kobayashi, A.; Xing, F.; Fukuda, K.; Hirota, S.; Sugai, T.; et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S.; et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/beta-catenin negative feedback loop. Stem. Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D. Farmer to pharmacist: Curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front. Chem. 2014, 2, 113. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, Z.; Fang, L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB-Snail signaling in breast cancer cells. Oncol. Rep. 2013, 29, 117–124. [Google Scholar] [PubMed]
- Belguise, K.; Guo, S.; Yang, S.; Rogers, A.E.; Seldin, D.C.; Sherr, D.H.; Sonenshein, G.E. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007, 67, 11742–11750. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.F.; Ma, H.Z.; Xin, H.L.; Feng, J.; Wen, A.D.; Li, Y. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. Int. J. Mol. Sci. 2013, 14, 15655–15668. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Valente, C.M.; Tinelli, A.; Siciliano, C.; Lorusso, V.; Acierno, R.; Giovinazzo, G.; Santino, A.; Storelli, C.; Maffia, M. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011, 310, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine 2015, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Park, J.; Sharma, A.R.; Jung, J.S.; Kim, H.; Chakraborty, C.; Song, D.K.; Lee, S.S.; Nam, J.S. Methoxy poly(ethylene glycol)-poly(lactide) nanoparticles encapsulating quercetin act as an effective anticancer agent by inducing apoptosis in breast cancer. Pharm. Res. 2015, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Barrajon-Catalan, E.; Nicolosi, S.; Cicirata, F.; Micol, V. Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res. Treat. 2013, 141, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Verderio, P.; Bonetti, P.; Colombo, M.; Pandolfi, L.; Prosperi, D. Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules 2013, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, R.; Zhong, Z.; Shi, Z.; Chen, M.; Wang, Y. Epigallocatechin-3-gallate potentiates the effect of curcumin in inducing growth inhibition and apoptosis of resistant breast cancer cells. Am. J. Chin. Med. 2014, 42, 1279–1300. [Google Scholar] [CrossRef] [PubMed]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ERα-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2008, 122, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Childs, B.H.; Pegram, M. Triple negative breast cancer: Unmet medical needs. Breast Cancer Res. Treat. 2011, 125, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, R.; Braicu, C.; Berindan-Neagoe, I. Another review on triple negative breast cancer. Are we on the right way towards the exit from the labyrinth? Breast 2013, 22, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Walerych, D.; Napoli, M.; Collavin, L.; del Sal, G. The rebel angel: Mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 2012, 33, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Freed-Pastor, W.A.; Prives, C. Mutant p53: One name, many proteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pileczki, V.; Pop, L.; Petric, R.C.; Chira, S.; Pointiere, E.; Achimas-Cadariu, P.; Berindan-Neagoe, I. Dual targeted therapy with p53 siRNA and Epigallocatechingallate in a triple negative breast cancer cell model. PLoS ONE 2015, 10, e0120936. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, G.A.; Al-Abd, A.M.; Tadros, M.G.; Al-Abbasi, F.A.; Khalifa, A.E.; Abdel-Naim, A.B. The chemomodulatory effects of resveratrol and didox on herceptin cytotoxicity in breast cancer cell lines. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Yamabe, N.; Zhu, B.T. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur. J. Cancer 2010, 46, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Murrill, W.B.; Brown, N.M.; Zhang, J.X.; Manzolillo, P.A.; Barnes, S.; Lamartiniere, C.A. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis 1996, 17, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; MacDonald, R.S. Soy isoflavones increase latency of spontaneous mammary tumors in mice. J. Nutr. 2002, 132, 3186–3190. [Google Scholar] [PubMed]
- Ju, Y.H.; Allred, C.D.; Allred, K.F.; Karko, K.L.; Doerge, D.R.; Helferich, W.G. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J. Nutr. 2001, 131, 2957–2962. [Google Scholar] [PubMed]
- Ju, Y.H.; Doerge, D.R.; Allred, K.F.; Allred, C.D.; Helferich, W.G. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002, 62, 2474–2477. [Google Scholar] [PubMed]
- Kijkuokool, P.; Parhar, I.S.; Malaivijitnond, S. Genistein enhances N-nitrosomethylurea-induced rat mammary tumorigenesis. Cancer Lett. 2006, 242, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, A.; Wang, M.; Olivo, S.; DeAssis, S.; Gustafsson, J.A.; Khan, G.; Hilakivi-Clarke, L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis 2004, 25, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.I.; Lantvit, D.; Hawthorne, M.; Xu, X.; van Breemen, R.B.; Pezzuto, J.M. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr. Cancer 2001, 41, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lamartiniere, C.A.; Wang, J.; Smith-Johnson, M.; Eltoum, I.E. Daidzein: Bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol. Sci. 2002, 65, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar] [PubMed]
- Whitsett, T.; Carpenter, M.; Lamartiniere, C.A. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J. Carcinog. 2006, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Johnson, J.A.; Gould, M.N.; Tanner, M.A. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988, 48, 5754–5758. [Google Scholar] [PubMed]
- Singh, B.; Mense, S.M.; Bhat, N.K.; Putty, S.; Guthiel, W.A.; Remotti, F.; Bhat, H.K. Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol. Appl. Pharmacol. 2010, 247, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pichardo, L.; Martinez-Montemayor, M.M.; Martinez, J.E.; Wall, K.M.; Cubano, L.A.; Dharmawardhane, S. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin. Exp. Metastasis 2009, 26, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, S.A.; Wilson, S.M.; Postenka, C.O.; Al-Katib, W.; Tuck, A.B.; Chambers, A.F. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005, 65, 3396–3403. [Google Scholar] [PubMed]
- Farhangi, B.; Alizadeh, A.M.; Khodayari, H.; Khodayari, S.; Dehghan, M.J.; Khori, V.; Heidarzadeh, A.; Khaniki, M.; Sadeghiezadeh, M.; Najafi, F. Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur. J. Pharmacol. 2015, 758, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Belosay, A.; Hartman, J.A.; Song, H.; Zhang, Y.; Wang, W.; Doerge, D.R.; Helferich, W.G. Dietary soy isoflavones increase metastasis to lungs in an experimental model of breast cancer with bone micro-tumors. Clin. Exp. Metastasis 2015, 32, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Longatto Filho, A.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and breast cancer. J. Oncol. 2010, 23, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NF-κB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Lawenda, B.D.; Smith, D.E.; Xu, L.; Niemierko, A.; Silverstein, J.R.; Boucher, Y.; Kashiwagi, S.; Held, K.D.; Jain, R.K.; Loeffler, J.S.; et al. Do the dietary supplements epigallocatechin gallate or vitamin e cause a radiomodifying response on tumors in vivo? A pilot study with murine breast carcinoma. J. Soc. Integr. Oncol. 2007, 5, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jin, S.; Zhang, Q. Antitumor and antiangiogenic activity of soy phytoestrogen on 7,12-dimethylbenz[α]anthracene-induced mammary tumors following ovariectomy in Sprague-Dawley rats. J. Food Sci. 2009, 74, H237–H242. [Google Scholar] [CrossRef] [PubMed]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. Biomed. Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Dubey, P.; Roten, S.; Kute, T. Effects of soy extracts on the growth of herceptin sensitive and resistant breast cancer cells in vitro and in vivo. J. N. C. Acad. Sci. 2006, 122, 19–28. [Google Scholar]
- Du, M.; Yang, X.; Hartman, J.A.; Cooke, P.S.; Doerge, D.R.; Ju, Y.H.; Helferich, W.G. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in athymic nude mice. Carcinogenesis 2012, 33, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Effect of soy isoflavones on the growth of human breast tumors: Findings from preclinical studies. Food Sci. Nutr. 2014, 2, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Blancafort, A.; Giro-Perafita, A.; Oliveras, G.; Palomeras, S.; Turrado, C.; Campuzano, O.; Carrion-Salip, D.; Massaguer, A.; Brugada, R.; Palafox, M.; et al. Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs. PLoS ONE 2015, 10, e0131241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (−)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. 2010, 12, R8. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, S.H.; Price, J.E.; Kim, L.S. Curcumin suppresses the paclitaxel-induced nuclear factor-κB in breast cancer cells and potentiates the growth inhibitory effect of paclitaxel in a breast cancer nude mice model. Breast J. 2009, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hoag, S.W.; Hussain, A.S. The impact of formulation on bioavailability: Summary of workshop discussion. J. Nutr. 2001, 131 (Suppl.), 1389S–1391S. [Google Scholar] [PubMed]
- Heaney, R.P. Factors influencing the measurement of bioavailability, taking calcium as a model. J. Nutr. 2001, 131 (Suppl.), 1344S–1348S. [Google Scholar] [PubMed]
- Srinivasan, V.S. Bioavailability of nutrients: A practical approach to in vitro demonstration of the availability of nutrients in multivitamin-mineral combination products. J. Nutr. 2001, 131 (Suppl.), 1349S–1350S. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl.), 230S–242S. [Google Scholar] [PubMed]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; van Trijp, J.M.; Buysman, M.N.; van der Gaag, M.S.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Maenpaa, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [PubMed]
- Richelle, M.; Tavazzi, I.; Enslen, M.; Offord, E.A. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999, 53, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [PubMed]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Shelnutt, S.R.; Cimino, C.O.; Wiggins, P.A.; Ronis, M.J.; Badger, T.M. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am. J. Clin. Nutr. 2002, 76, 588–594. [Google Scholar] [PubMed]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131 (Suppl.), 1362S–1375S. [Google Scholar] [PubMed]
- Shu, X.O.; Jin, F.; Dai, Q.; Wen, W.; Potter, J.D.; Kushi, L.H.; Ruan, Z.; Gao, Y.T.; Zheng, W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 483–488. [Google Scholar]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S.; et al. Soy intake and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Verheus, M.; van Gils, C.H.; Keinan-Boker, L.; Grace, P.B.; Bingham, S.A.; Peeters, P.H. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007, 25, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Seely, D.; Flower, G.; Skidmore, B.; Fernandes, R.; Vadeboncoeur, S.; Kennedy, D.; Cooley, K.; Wong, R.; Sagar, S.; et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS ONE 2013, 8, e81968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.O.; Huang, Y.B.; Gao, Y.; Chen, C.; Yan, Y.; Dai, H.J.; Song, F.J.; Wang, Y.G.; Wang, P.S.; Chen, K.X. Association between dietary factors and breast cancer risk among Chinese females: Systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2014, 15, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.C.; Baltar, V.T.; Marchioni, D.M. Breast cancer and dietary patterns: A systematic review. Nutr. Rev. 2014, 72, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Takata, Y.; Franke, A.A.; Williams, A.E.; Murphy, S.P. A 2-year soy intervention in premenopausal women does not change mammographic densities. J. Nutr. 2004, 134, 3089–3094. [Google Scholar] [PubMed]
- Maskarinec, G.; Williams, A.E.; Carlin, L. Mammographic densities in a one-year isoflavone intervention. Eur. J. Cancer Prev. 2003, 12, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; McCaskill-Stevens, W.; Lampe, J.W. Addressing the soy and breast cancer relationship: Review, commentary, and workshop proceedings. J. Natl. Cancer Inst. 2006, 98, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Guha, N.; Kwan, M.L.; Quesenberry, C.P., Jr.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The Life After Cancer Epidemiology study. Breast Cancer Res. Treat. 2009, 118, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Wan, P.; Hankin, J.; Tseng, C.C.; Yu, M.C.; Pike, M.C. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 2002, 23, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; Roberts, S.A.; Howell, A.; Potten, C.S.; Bundred, N.J. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 1998, 68 (Suppl.), 1431S–1435S. [Google Scholar] [PubMed]
- Frankenfeld, C.L.; McTiernan, A.; Aiello, E.J.; Thomas, W.K.; LaCroix, K.; Schramm, J.; Schwartz, S.M.; Holt, V.L.; Lampe, J.W. Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1156–1162. [Google Scholar]
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wahala, K.; Thomas, W.K.; Lampe, J.W. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351. [Google Scholar] [PubMed]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; la Vecchia, C. Resveratrol and breast cancer risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.M.; Shu, X.O.; Gao, Y.T.; Dai, Q.; Yu, H.; Cheng, J.R.; Jin, F.; Zheng, W. Correlation of blood sex steroid hormones with body size, body fat distribution, and other known risk factors for breast cancer in post-menopausal Chinese women. Cancer Causes Control 2004, 15, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar] [PubMed]
- Ursin, G.; London, S.; Stanczyk, F.Z.; Gentzschein, E.; Paganini-Hill, A.; Ross, R.K.; Pike, M.C. Urinary 2-hydroxyestrone/16α-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1999, 91, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.M.; Kurzer, M.S. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Inoue, M.; Sasazuki, S.; Miura, T.; Sawada, N.; Yamaji, T.; Shimazu, T.; Willett, W.C.; Tsugane, S. Plasma tea polyphenol levels and subsequent risk of breast cancer among Japanese women: A nested case-control study. Breast Cancer Res. Treat. 2010, 124, 827–834. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, M.-M.; Nagy, P.; Szöllősi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578-22620. https://doi.org/10.3390/molecules201219864
Mocanu M-M, Nagy P, Szöllősi J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules. 2015; 20(12):22578-22620. https://doi.org/10.3390/molecules201219864
Chicago/Turabian StyleMocanu, Maria-Magdalena, Péter Nagy, and János Szöllősi. 2015. "Chemoprevention of Breast Cancer by Dietary Polyphenols" Molecules 20, no. 12: 22578-22620. https://doi.org/10.3390/molecules201219864