Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives
Abstract
:1. Introduction
2. Results and Discussion
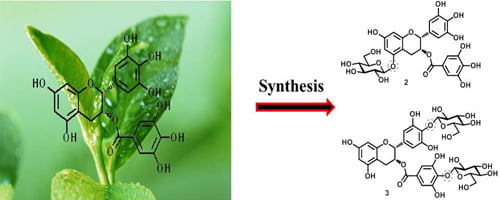
2.1. Chemical Synthesis and Structural Determination of EGCG Glycosides
2.2. Anticancer Activity
2.3. Antioxidant Activity
2.4. Stability Investigation
2.5. Effects of Glycosylation on Water Solubility
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Synthesis of EGCG-G1 and EGCG-G2
3.3. Cell Culture and Cytotoxicity Assay
3.4. DPPH Radical-Scavenging Assay
3.5. Stability Assay
3.6. Water Solubility Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Sun, C.; Butler, L.M. Tea and cancer prevention: Epidemiological studies. Pharmacol. Res. 2011, 64, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, L.L.; Zhang, X.; Wu, Z.F.; Weng, P.F. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia 2015, 104, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Gimenez, R.; Lopez, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidant. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.W.; Bae, S.M.; Kim, Y.W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Yeo, M.; Choue, J.S.; Jin, J.H.; Park, S.J.; Cheong, J.Y.; Lee, K.J.; Kim, J.H.; Hahm, K.B. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter 2004, 9, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin, C.C.; Lin, J.K.; Chen, W.J. Tea polyphenol (−)-epigallocatechin-3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. J. Agric. Food Chem. 2007, 55, 5030–5037. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.C.; Rosengren, R.J. The combination of raloxifene and epigallocatechin gallate suppresses growth and induces apoptosis in MDA-MB-231 cells. Life Sci. 2008, 82, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.H.; Ku, C.Y.; Ho, C.T.; Chen, C.S.; Huang, C.S.; Lee, C.H.; Chen, L.C.; Pan, M.H.; Chang, H.W.; Chang, C.S.; et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced α9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol. Nutr. Food Res. 2011, 55, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, X.W.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin-3-gallate (EGCG) in invasive breast cancer cells. J. Biol. Chem. 2006, 16, 10865–10875. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Matsudo, T.; Saitoh, M.; Horiuchi, T.; Sekine, H. Enzymatic syntheses of two stable (−)-epigallocatechin gallateglucosides by sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1995, 59, 2167–2169. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Wong, I.L.K.; Wang, B.C.; Yuan, J.; Duan, L.X.; Liu, Z.; Liu, T.; Li, X.M.; Hu, X.; Zhang, X.Y.; Jiang, T.; et al. Potent and Nontoxic Chemosensitizer of P-glycoprotein-mediated Multidrug Resistance in Cancer: Synthesis and Evaluation of Methylated Epigallocatechin, Gallocatechin, and Dihydromyricetin Derivatives. J. Med. Chem. 2015, 58, 4529–4549. [Google Scholar] [CrossRef] [PubMed]
- Qsanai, K.; Landis-Piwowar, K.R.; Dou, Q.P.; Chan, T.H. A para-amino substituent on the D-ring of green tea polyphenol epigallocatechin-3-gallate as a novel proteasome inhibitor and cancer cell apoptosis inducer. Bioorg. Med. Chem. 2007, 15, 5076–5082. [Google Scholar]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.C.; Chan, A.S.C.; Dou, Q.P.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorg. Med. Chem. Lett. 2004, 12, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.L.; Li, X.M.; Yuan, J.; Chen, D.; Jiang, T.; Dou, Q.P.; Chan, T.H.; Wan, S.B. Semisynthesis of fluoro-substituted benzoates of epi-gallocatechin. Synth. Commun. 2012, 42, 3524–3531. [Google Scholar] [CrossRef]
- Anderson, J.C.; Headley, C.; Stapleton, P.D.; Taylor, P.W. Synthesis and antibacterial activity of hydrolytically stable (−)-epicatechin gallate analogues for the modulation of β-lactam resistance in Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2005, 15, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Unnadkat, N.R.; Elias, R.J. Oxidative stability of (−)-epigallocatechin gallate in the presence of thiols. J. Agric. Food Chem. 2012, 60, 10815–10821. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorg. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.H.; Lee, J.H.; Ahn, J.S.; Nam, S.H.; Oh, D.K.; Park, D.H.; Chung, H.J.; Kang, S.; Day, D.F.; Kim, D. Synthesis, structure analyses, and characterization of novel epigallocatechin gallate (EGCG) glycosides using the glucansucrase from Leuconostoc mesenteroides B-1299CB. J. Agric. Food Chem. 2006, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Ariga, T.; Matsudo, T.; Sekine, H. The syntheses of catechin-glucosides by transglycosylation with Leuconostoc mesenteroides sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1993, 57, 2010–2015. [Google Scholar] [CrossRef]
- Sato, T.; Nakagawa, H.; Kurosu, J.; Yoshida, K.; Tsugane, T.; Shimura, S.; Kirimura, K.; Kino, K.; Usami, S. R-Anomerselective glucosylation of (+)-catechin by the crude enzyme, showing glucosyl transfer activity, of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 2000, 90, 625–630. [Google Scholar] [CrossRef]
- Nakano, H.; Hamayasu, K.; Nakagawa, K.; Tabata, A.; Fujita, K.; Hara, K.; Kiso, T.; Murakami, H.; Kitahata, S. Transglycosylation of hydroquinone and epicatechin by β-fructofuranosidase from Arthrobacter sp. J. Appl. Glycosci. 2002, 49, 115–121. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [PubMed]
- Arafa, H.M.M. Possible contribution of β-glycosidases and caspases in the cytotoxicity of novel glycoconjugates in colon cancer cells. Investig. New Drugs 2010, 28, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Fukami, H.; Nakao, M.; Noguchi, A. Method for Glycosylation of Flavonoid. U.S. Patent 2010/0256345 A1, 17 April 2013. [Google Scholar]
- Moon, Y.H.; Kim, G.K.; Lee, J.H.; Jin, X.J.; Kim, D.W.; Kim, D. Enzymatic synthesis and characterization of novel epigallocatechin gallate glucosides. J. Mol. Catal. B Enzym. 2006, 40, 1–7. [Google Scholar] [CrossRef]
- Lu, L.D.; Ning, Q.; Lu, Q.B. Antioxidant Induces DNA Damage, Cell Death and Mutagenicity in Human Lung and Skin Normal Cells. Sci. Rep. 2013, 3, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Pate, M.S.; Wylie, R.C.; Tollefsbol, T.O.; Katiyar, S.K. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 2004, 24, 703–710. [Google Scholar] [PubMed]
- Scandlyn, M.J.; Stuart, E.C.; Somers-Edgar, T.J.; Menzies, A.R.; Rosengren, R.J. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br. J. Cancer 2008, 99, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Utenova, B.T.; Malterud, K.E.; Rise, F. Antioxidant activity of O-protected derivatives of (−)-epigallocatechin-3-gallate: Inhibition of soybean and rabbit 15-lipoxygenases. ARKIVOC 2007, 9, 6–16. [Google Scholar]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Czechura, P.; Tam, R.Y.; Dimitrijevic, E.; Murphy, A.V.; Ben, R.N. The Importance of Hydration for Inhibiting Ice Recrystallization with C-Linked Antifreeze Glycoproteins. J. Am. Chem. Soc. 2008, 130, 2928–2929. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.V.; Maeda, T.; Miyatake, K.; Morita, N. Total phenolic compounds and antioxidant capacity of wheat graded flours by polishing method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Li, D.; Park, S.H.; Shim, J.H.; Lee, H.S.; Tang, S.Y.; Park, C.S.; Park, K.H. In vitro enzymatic modification of puerarin to puerarin glycosides by maltogenic amylase. Carbohydr. Res. 2004, 339, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds EGCG (1), EGCG-G1 (2), and EGCG-G2 (3) are available from the authors.





| C Position | EGCG-G1 (δ1) | EGCG-G2 (δ2) | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| EGCG | ||||
| 2 | 77.3 | 5.03 (s) | 77.2 | 5.03 (s) |
| 3 | 69.3 | 5.37 (br s) | 69.4 | 5.38 (br s) |
| 4 | 25.6 | 2.89–2.69 (m) | 25.6 | 2.99–2.89 (m) |
| 2.58–2.49 (m) | 2.70–2.66 (m) | |||
| 5 | 156.7 | 156.7 | ||
| 6 | 95.7 | 5.94 (d, J = 2.4 Hz) | 95.7 | 5.94 (d, J = 2.4 Hz) |
| 7 | 155.3 | 155.3 | ||
| 8 | 94.2 | 5.84 (d, J = 2.4 Hz) | 94.3 | 5.84 (d, J = 2.4 Hz) |
| 9 | 156.5 | 156.5 | ||
| 10 | 92.0 | 92.1 | ||
| 1′ | 125.6 | 125.6 | ||
| 2′/6′ | 105.6 | 6.52 (s) | 105.8 | 6.51 (s) |
| 3′/5′ | 150.3 | 150.3 | ||
| 4′ | 135.6 | 135.6 | ||
| 1′′ | 132.6 | 132.6 | ||
| 2′′/6′′ | 108.6 | 6.82 (s) | 108.6 | 6.80 (s) |
| 3′′/5′′ | 149.8 | 149.8 | ||
| 4′′ | 137.1 | 137.1 | ||
| COO- | 164.7 | 164.7 | ||
| Glucose (-G1) | ||||
| 1′′′ | 106.0 | 4.87 (d, J = 9.0 Hz) | 106.0 | 4.70 (d, J = 9.5 Hz) |
| 2′′′ | 75.1 | 3.33–3.15 (m) | 75.8 | 3.30–3.22 (m) |
| 3′′′ | 76.4 | 5.25–5.24 (m) | 76.7 | 5.14–5.13 (m) |
| 4′′′ | 73.6 | 3.33–3.15 (m) | 73.6 | 3.30–3.22 (m) |
| 5′′′ | 77.2 | 4.59–4.57 (m) | 77.1 | 4.56–4.55 (m) |
| 6′′′ | 60.4 | 3.60–3.58 (m) | 60.4 | 3.61–3.57 (m) |
| Glucose (-G2) | ||||
| 1′′′′ | 104.6 | 4.46 (d, J = 9.5 Hz) | ||
| 2′′′′ | 75.7 | 3.30–3.22 (m) | ||
| 3′′′′ | 76.1 | 5.24–5.23 (m) | ||
| 4′′′′ | 73.6 | 3.30–3.22 (m) | ||
| 5′′′′ | 77.1 | 4.61–4.60 (m) | ||
| 6′′′′ | 61.2 | 3.61–3.57 (m) | ||
| Compound | Solubility in Water a (mM) | Relative Solubility |
|---|---|---|
| EGCG | 16.05 ± 1.23 | 1 |
| EGCG-G1 | 240.93 ± 1.91 | 15 |
| EGCG-G2 | 504.73 ± 0.57 | 31 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.; Hu, J.-M.; Huang, Y.-W.; Wu, X.-Y.; Zi, C.-T.; Wang, X.-J.; Sheng, J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules 2016, 21, 620. https://doi.org/10.3390/molecules21050620
Zhang X, Wang J, Hu J-M, Huang Y-W, Wu X-Y, Zi C-T, Wang X-J, Sheng J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules. 2016; 21(5):620. https://doi.org/10.3390/molecules21050620
Chicago/Turabian StyleZhang, Xin, Jing Wang, Jiang-Miao Hu, Ye-Wei Huang, Xiao-Yun Wu, Cheng-Ting Zi, Xuan-Jun Wang, and Jun Sheng. 2016. "Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives" Molecules 21, no. 5: 620. https://doi.org/10.3390/molecules21050620