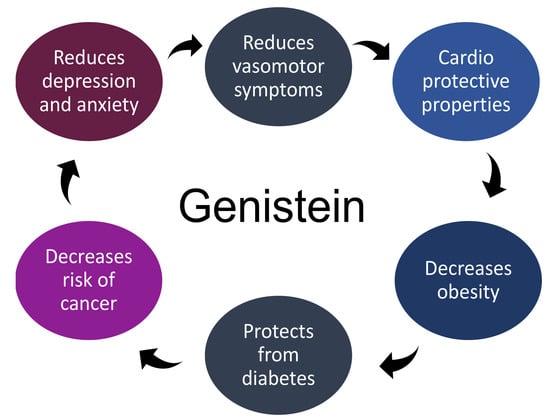
Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases
Abstract
:1. Introduction
2. Genistein and Its Therapeutic Effects
2.1. Effect of Genistein in Treating Postmenopausal Vasomotor Symptoms
2.2. Cardioprotective Effects of Genistein
2.3. Role of Genistein in Obesity
2.4. Role of Genistein in Diabetes
2.5. Role of Genistein in Cancer
2.6. Antidepressant and Anxiolytic Effects of Genistein
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thurston, R.C.; Chang, Y.; Mancuso, P.; Matthews, K.A. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: Findings from the Study of Women’s Health Across the Nation. Fertil. Steril. 2013, 100, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alonso, A.M.; Cuadros, J.L.; Chedraui, P.; Mendoza, M.; Cuadros, A.M.; Pérez-López, F.R. Obesity is related to increased menopausal symptoms among Spanish women. Menopause Int. 2010, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinicaland Economic Aspects ofOsteoporosis and Osteoarthritis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin. Exp. Res. 2019, 31, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.A.; Almaria, M.J.G. Postmenopausal endometriosis: Drawing a clearer clinical picture. Climacteric 2018, 21, 249–255. [Google Scholar] [CrossRef]
- Mintziori, G.; Lambrinoudaki, I.; Goulis, D.G.; Ceausu, I.; Depypere, H.; Erel, C.T.; Perez-Lopez, F.R.; Schenck-Gustafsson, K.; Simoncini, T.; Tremollieres, F.; et al. EMAS position statement: Non-hormonal management of menopausal vasomotor symptoms. Maturitas 2015, 81, 410–413. [Google Scholar] [CrossRef]
- Franco, O.H.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2554–2563. [Google Scholar] [CrossRef]
- Su, B.Y.; Tung, T.H.; Chien, W.H. Effects of Phytoestrogens on Depressive Symptoms in Climacteric Women: A Meta-Analysis of Randomized Controlled Trials. J. Altern. Complement. Med. 2018, 24, 850–851. [Google Scholar] [CrossRef]
- Estrada-Camarena, E.; López-Rubalcava, C.; Valdés-Sustaita, B.; Sinhue, A.M.G.; González-Trujano, E.M. Use of Phytoestrogens for the Treatment of Psychiatric Symptoms Associated with Menopause Transition. In A Multidisciplinary Look at Menopause; Rodríguez-Landa, J.F., Cueto-Escobedo, J., Eds.; InTech Open: Rijeka, Croatia, 2017; pp. 81–109. [Google Scholar]
- Rodríguez-Landa, J.F.; Puga-Olguín, A.; Germán-Ponciano, L.J.; Olmos-Vázquez, O.J.; Bernal-Morales, B. Chapter 5-Phytoestrogens as Potential Therapeutic Agents for the Treatment of Anxiety and Affective Disorders. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 133–159. [Google Scholar]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]
- Sakla, M.S.; Shenouda, N.S.; Ansell, P.J.; Macdonald, R.S.; Lubahn, D.B. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine 2007, 32, 69–78. [Google Scholar] [CrossRef]
- Johnson, A.; Roberts, L.; Elkins, G. Complementary and Alternative Medicine for Menopause. J. Evid. Based Integr. Med. 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, W.; Yang, X. Soybean soluble polysaccharide enhances absorption of soybean genistein in mice. Food Res. Int. 2018, 103, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, M.M.K.; Mahmoud, S.S.; El-Sayed, S.H.; Rizk, E.M.A.; Raafat, A.; Negm, M.S.I. Impact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 2018, 185, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhauser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid. Med. Cell Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xing, K.; Li, G.; Liu, D.; Guo, Y. Dietary Genistein Alleviates Lipid Metabolism Disorder and Inflammatory Response in Laying Hens With Fatty Liver Syndrome. Front. Physiol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.R.; Feng, X.Q.; Li, N.; Qu, J.X.; Feng, L.; Chen, L.; Chen, W.F. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine 2018, 43, 11–20. [Google Scholar] [CrossRef]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef]
- Huang, J.; Nasr, M.; Kim, Y.; Matthews, H.R. Genistein inhibits protein histidine kinase. J. Biol. Chem. 1992, 267, 15511–15515. [Google Scholar]
- Si, H.; Liu, D. Phytochemical genistein in the regulation of vascular function: New insights. Curr. Med. Chem. 2007, 14, 2581–2589. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Doerge, D.R. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 2000, 168, 244–252. [Google Scholar] [CrossRef]
- Nogowski, L.; Nowak, K.W.; Kaczmarek, P.; Mackowiak, P. The influence of coumestrol, zearalenone, and genistein administration on insulin receptors and insulin secretion in ovariectomized rats. J. Recept. Signal. Transduct. Res. 2002, 22, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Shinoda, S.; Toyoshima, S.; Nakazawa, H.; Makino, T.; Nakajin, S. Effects of flavonoid phytochemicals on cortisol production and on activities of steroidogenic enzymes in human adrenocortical H295R cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 355–363. [Google Scholar] [CrossRef]
- Ohno, S.; Nakajima, Y.; Inoue, K.; Nakazawa, H.; Nakajin, S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 2003, 74, 733–742. [Google Scholar] [CrossRef]
- Guo, P.P.; Li, P.; Zhang, X.H.; Liu, N.; Wang, J.; Chen, D.D.; Sun, W.J.; Zhang, W. Complementary and alternative medicine for natural and treatment-induced vasomotor symptoms: An overview of systematic reviews and meta-analyses. Complement. Ther. Clin. Pract. 2019, 36, 181–194. [Google Scholar] [CrossRef]
- Mareti, E.; Abatzi, C.; Vavilis, D.; Lambrinoudaki, I.; Goulis, D.G. Effect of oral phytoestrogens on endometrial thickness and breast density of perimenopausal and postmenopausal women: A systematic review and meta-analysis. Maturitas 2019, 124, 81–88. [Google Scholar] [CrossRef]
- Mukund, V.; Behera, S.K.; Alam, A.; Nagaraju, G.P. Molecular docking analysis of nuclear factor-κB and genistein interaction in the context of breast cancer. Bioinformation 2019, 15, 11–17. [Google Scholar] [CrossRef]
- Pons, D.G.; Vilanova-Llompart, J.; Gaya-Bover, A.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. The phytoestrogen genistein affects inflammatory-related genes expression depending on the ERα/ERβ ratio in breast cancer cells. Int. J. Food Sci. Nutr. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Braxas, H.; Rafraf, M.; Hasanabad, S.K.; Jafarabadi, M.A. Effectiveness of genistein supplementation on metabolic factors and antioxidant status in postmenopausal women with type-2 diabetes mellitus. Can. J. Diabetes 2019, 43, 490–497. [Google Scholar] [CrossRef]
- Nayeem, F.; Chen, N.W.; Nagamani, M.; Anderson, K.E.; Lu, L.J. Daidzein and genistein have differential effects in decreasing whole body bone mineral density but had no effect on hip and spine density in premenopausal women: A 2-year randomized, double-blind, placebo-controlled study. Nutr. Res. 2019, 68, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, A.; Sakakibara, H.; Zhou, W.; Yoshioka, M.; Ohsumi, M.; Shimoi, K.; Yokogoshi, H. Genistein regulated serotonergic activity in the hippocampus of ovariectomized rats under forced swimming stress. Biosci. Biotechnol. Biochem. 2010, 74, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Caretto, M.; Giannini, A.; Simoncini, T.; Genazzani, A.R. Menopause and Ageing. In Reproductive Medicine for Clinical Practice. Reproductive Medicine for Clinicians; Schenker, J., Sciarra, J., Mettler, L., Genazzani, A., Birkhaeuser, M., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 177–189. [Google Scholar]
- Gartoulla, P.; Bell, R.J.; Worsley, R.; Davis, S.R. Menopausal vasomotor symptoms are associated with poor self-assessed work ability. Maturitas 2016, 87, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zeleke, B.M.; Bell, R.J.; Billah, B.; Davis, S.R. Vasomotor and sexual symptoms in older Australian women: A cross-sectional study. Fertil. Steril. 2016, 105, 149–155.e1. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Soy product intake and hot flashes in Japanese women: Results from a community-based prospective study. Am. J. Epidemiol. 2001, 153, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, A.; Marini, H.; Bitto, A.; Altavilla, D.; Squadrito, G.; Romeo, A.; Adamo, E.B.; Marini, R.; D’Anna, R.; Corrado, F.; et al. Effects of genistein on hot flushes in early postmenopausal women: A randomized, double-blind EPT-and placebo-controlled study. Menopause 2004, 11, 400–414. [Google Scholar] [CrossRef]
- Usategui-Martin, R.; Perez-Alonso, M.; Socorro-Briongos, L.; Ruiz-Mambrilla, M.; De Luis, D.; Linares, L.; Calero-Paniagua, I.; Duenas-Laita, A.; Perez-Castrillon, J.L. Estrogen receptor genes polymorphisms determine serum lipid profile in healthy postmenopausal women treated with calcium, vitamin D, and genistein. J. Cell Biochem. 2019, 120, 13115–13120. [Google Scholar] [CrossRef]
- Teede, H.J. Sex hormones and the cardiovascular system: Effects on arterial function in women. Clin. Exp. Pharmacol Physiol 2007, 34, 672–676. [Google Scholar] [CrossRef]
- Atteritano, M.; Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Mazzaferro, S.; D’Anna, R.; Cannata, M.L.; Gaudio, A.; et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: A two-year randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 3068–3075. [Google Scholar] [CrossRef]
- Irace, C.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; Arcoraci, V.; Minutoli, L.; Di Benedetto, A.; Di Vieste, G.; et al. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur. J. Clin. Investig. 2013, 43, 1025–1031. [Google Scholar] [CrossRef]
- Crisafulli, A.; Altavilla, D.; Marini, H.; Bitto, A.; Cucinotta, D.; Frisina, N.; Corrado, F.; D’Anna, R.; Squadrito, G.; Adamo, E.B.; et al. Effects of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women. Menopause 2005, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Deodato, B.; Altavilla, D.; Squadrito, G.; Campo, G.M.; Arlotta, M.; Minutoli, L.; Saitta, A.; Cucinotta, D.; Calapai, G.; Caputi, A.P.; et al. Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia-reperfusion injury. Br. J. Pharmacol. 1999, 128, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelska, K.; Nogowski, L. Genistein--a dietary compound inducing hormonal and metabolic changes. J. Steroid Biochem. Mol. Biol. 2007, 105, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jayagopal, V.; Albertazzi, P.; Kilpatrick, E.S.; Howarth, E.M.; Jennings, P.E.; Hepburn, D.A.; Atkin, S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002, 25, 1709–1714. [Google Scholar] [CrossRef]
- Bitto, A.; Polito, F.; Atteritano, M.; Altavilla, D.; Mazzaferro, S.; Marini, H.; Adamo, E.B.; D’Anna, R.; Granese, R.; Corrado, F.; et al. Genistein aglycone does not affect thyroid function: Results from a three-year, randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2010, 95, 3067–3072. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef]
- De Gregorio, C.; Marini, H.; Alibrandi, A.; Di Benedetto, A.; Bitto, A.; Adamo, E.B.; Altavilla, D.; Irace, C.; Di Vieste, G.; Pancaldo, D.; et al. Genistein Supplementation and Cardiac Function in Postmenopausal Women with Metabolic Syndrome: Results from a Pilot Strain-Echo Study. Nutrients 2017, 9, 584. [Google Scholar] [CrossRef]
- Villa, P.; Costantini, B.; Suriano, R.; Perri, C.; Macri, F.; Ricciardi, L.; Panunzi, S.; Lanzone, A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: Relationship with the metabolic status. J. Clin. Endocrinol. Metab. 2009, 94, 552–558. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Canete, R.R.; Gil, A. Adiponectin, the missing link in insulin resistance and obesity. Clin. Nutr. 2004, 23, 963–974. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Shin, S.J.; Lee, Y.J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Puga-Olguín, A.; Rodríguez-Landa, J.F.; Rovirosa-Hernández, M.J.; Germán-Ponciano, L.J.; Caba, M.; Meza, E.; Guillén-Ruiz, G.; Olmos-Vázquez, O.J. Long-term ovariectomy increases anxiety- and despair-like behaviors associated with lower Fos immunoreactivity in the lateral septal nucleus in rats. Behav. Brain Res. 2019, 360, 185–195. [Google Scholar]
- Al-Nakkash, L.; Markus, B.; Batia, L.; Prozialeck, W.C.; Broderick, T.L. Genistein induces estrogen-like effects in ovariectomized rats but fails to increase cardiac GLUT4 and oxidative stress. J. Med. Food 2010, 13, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Lee, S.H. Genistein, a soy isoflavone, is a potent alpha-glucosidase inhibitor. FEBS Lett. 2001, 501, 84–86. [Google Scholar] [CrossRef]
- García-Jiménez, C.; Benito, B.; Jolin, T.; Santisteban, P. Insulin regulation of malic enzyme gene expression in rat liver: Evidence for nuclear proteins that bind to two putative insulin response elements. Mol. Endocrinol. 1994, 8, 1361–1369. [Google Scholar] [CrossRef]
- Khandelwal, R.L.; Pugazhenthi, S. In vivo effects of vanadate on hepatic glycogen metabolizing and lipogenic enzymes in insulin-dependent and insulin-resistant diabetic animals. Mol. Cell Biochem. 1995, 153, 87–94. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L. Soy consumption and prostate cancer risk in men: A revisit of a meta-analysis. Am. J. Clin. Nutr. 2009, 89, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.W.; Kim, S.Y.; Jee, S.H.; Kim, Y.N.; Nam, C.M. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr. Cancer 2009, 61, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, K.; Popiolkiewicz, J.; Krzeczynski, P.; Ramza, J.; Pucko, W.; Zegrocka-Stendel, O.; Boryski, J.; Skierski, J.S.; Mazurek, A.P.; Grynkiewicz, G. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004, 203, 59–69. [Google Scholar] [CrossRef]
- Popiolkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs-genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Gu, Y.; Zhu, C.F.; Iwamoto, H.; Chen, J.S. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J. Gastroenterol. 2005, 11, 6512–6517. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar]
- Chen, W.F.; Huang, M.H.; Tzang, C.H.; Yang, M.; Wong, M.S. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim. Biophys. Acta 2003, 1638, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.H.; Hung, M.C. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 2001, 3, 245–252. [Google Scholar] [CrossRef]
- Satoh, H.; Nishikawa, K.; Suzuki, K.; Asano, R.; Virgona, N.; Ichikawa, T.; Hagiwara, K.; Yano, T. Genistein, a soy isoflavone, enhances necrotic-like cell death in a breast cancer cell treated with a chemotherapeutic agent. Res. Commun. Mol. Pathol. Pharmacol. 2003, 113–114, 149–158. [Google Scholar]
- Zhou, B.P.; Liao, Y.; Xia, W.; Zou, Y.; Spohn, B.; Hung, M.C. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 2001, 3, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Chodon, D.; Ramamurty, N.; Sakthisekaran, D. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol. In Vitro 2007, 21, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 2017, 117, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoor, T.A.; Ramalho, R.M.; Luo, X.; Ramalhete, C.; Rodrigues, C.M.; Ferreira, M.J. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytother. Res. 2011, 25, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Chen, B.C.; Kao, S.T.; Liu, C.J.; Yeh, C.C. Genistein inhibits tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.C.; Chiang, P.C.; Li, T.K.; Hsu, J.L.; Lin, C.J.; Wang, S.W.; Peng, C.Y.; Guh, J.H. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem. Pharmacol. 2007, 73, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.M.; Lee, G.S.; An, B.S.; Jeung, E.B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 6. [Google Scholar] [CrossRef]
- Tian, T.; Li, J.; Li, B.; Wang, Y.; Li, M.; Ma, D.; Wang, X. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumour. Biol. 2014, 35, 4137–4145. [Google Scholar] [CrossRef]
- Hong, X.; Liu, P.; Liang, J.; Yun, F.U. Genistein induces apoptosis through upregulation of p53 signaling pathway. J. Trop. Med. 2008, 9, 4. [Google Scholar]
- El-Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008, 134, 1752–1763. [Google Scholar] [CrossRef]
- Yang, Y.; Zang, A.; Jia, Y.; Shang, Y.; Zhang, Z.; Ge, K.; Zhang, J.; Fan, W.; Wang, B. Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol. Lett. 2016, 12, 2189–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Acharya, B.R.; Bhattacharyya, B.; Chakrabarti, G. Genistein arrests cell cycle progression of A549 cells at the G(2)/M phase and depolymerizes interphase microtubules through binding to a unique site of tubulin. Biochemistry 2010, 49, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Shiau, R.J.; Chen, K.Y.; Wen, Y.D.; Chuang, C.H.; Yeh, S.L. Genistein and beta-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur. J. Nutr. 2010, 49, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Wu, L.P.; Li, K.H.; Liu, Y.P.; Xiang, R.; Zhang, S.B.; Zhu, L.Y.; Zhang, L.Y. Involvement of nuclear factor kappaB (NF-kappaB) in the downregulation of cyclooxygenase-2 (COX-2) by genistein in gastric cancer cells. J. Int. Med. Res. 2011, 39, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhang, G.Q.; Yang, Y.; Zhang, C.Y.; Fu, R.X.; Yang, Y.M. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr. Cancer 2013, 65, 1034–1041. [Google Scholar] [CrossRef]
- Yan, L.; Spitznagel, E.L.; Bosland, M.C. Soy consumption and colorectal cancer risk in humans: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 148–158. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.B.; Na, X.L.; Song, D.F.; Liu, Y. Blocking effects of genistein on cell proliferation and possible mechanism in human gastric carcinoma. World J. Gastroenterol. 2005, 11, 69–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp. Biol. Med. (Maywood) 2011, 236, 714–722. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Chen, H. Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS ONE 2012, 7, e40955. [Google Scholar] [CrossRef]
- Arai, N.; Strom, A.; Rafter, J.J.; Gustafsson, J.A. Estrogen receptor beta mRNA in colon cancer cells: Growth effects of estrogen and genistein. Biochem. Biophys. Res. Commun. 2000, 270, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Meisinger, J.; Van Thiel, D.H.; Zhang, Y.; Mobarhan, S. Effects of soybean extract on morphology and survival of Caco-2, SW620, and HT-29 cells. Nutr. Cancer 2002, 42, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, W.; Liu, F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004, 215, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Weber, C.R.; Wasland, K.; Savkovic, S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 2011, 11, 219. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.; Wang, R.; Wang, J.; Zhang, S.; Cai, X.; Wu, K.; Bergan, R.C.; Xu, L.; Fan, D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget 2015, 6, 3225–3239. [Google Scholar] [CrossRef]
- Chatzinikolaou, G.; Nikitovic, D.; Stathopoulos, E.N.; Velegrakis, G.A.; Karamanos, N.K.; Tzanakakis, G.N. Protein tyrosine kinase and estrogen receptor-dependent pathways regulate the synthesis and distribution of glycosaminoglycans/proteoglycans produced by two human colon cancer cell lines. Anticancer Res. 2007, 27, 4101–4106. [Google Scholar]
- Yan, G.R.; Xiao, C.L.; He, G.W.; Yin, X.F.; Chen, N.P.; Cao, Y.; He, Q.Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [CrossRef]
- Davis, N.M.; Sokolosky, M.; Stadelman, K.; Abrams, S.L.; Libra, M.; Candido, S.; Nicoletti, F.; Polesel, J.; Maestro, R.; D’Assoro, A.; et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: Possibilities for therapeutic intervention. Oncotarget 2014, 5, 4603–4650. [Google Scholar] [CrossRef]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef]
- Caetano, B.; Le Corre, L.; Chalabi, N.; Delort, L.; Bignon, Y.J.; Bernard-Gallon, D.J. Soya phytonutrients act on a panel of genes implicated with BRCA1 and BRCA2 oncosuppressors in human breast cell lines. Br. J. Nutr. 2006, 95, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; DeNardo, D.G.; Jacquot, Y.; Laios, I.; Vidal, D.S.; Zambrana, C.R.; Leclercq, G.; Brown, P.H. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res. Treat. 2006, 99, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Pernice, F.; Mazzaferro, S.; Mantuano, S.; Frisina, A.; D’Anna, R.; Cannata, M.L.; Bitto, A.; Squadrito, F.; Frisina, N.; et al. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur. J. Pharmacol. 2008, 589, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 2015, 54, 301–311. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Cueto-Escobedo, J.; Puga-Olguín, A.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V.; Santos-Torres, A. The Phytoestrogen Genistein Produces Similar Effects as 17beta-Estradiol on Anxiety-Like Behavior in Rats at 12 Weeks after Ovariectomy. Biomed. Res. Int. 2017, 2017, 9073816. [Google Scholar]
- Baffa, A.; Hohoff, C.; Baune, B.T.; Muller-Tidow, C.; Tidow, N.; Freitag, C.; Zwanzger, P.; Deckert, J.; Arolt, V.; Domschke, K. Norepinephrine and serotonin transporter genes: Impact on treatment response in depression. Neuropsychobiology 2010, 62, 121–131. [Google Scholar] [CrossRef]
- Shen, F.; Huang, W.L.; Xing, B.P.; Fang, X.; Feng, M.; Jiang, C.M. Genistein Improves the Major Depression through Suppressing the Expression of miR-221/222 by Targeting Connexin 43. Psychiatry Investig. 2018, 15, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Jia Jia, T.; Yee Woon, L.; Kumar Chellappan, D.; Candasamy, M.; Dua, K. Pharmacological Evaluation of Antidepressant-Like Effect of Genistein and Its Combination with Amitriptyline: An Acute and Chronic Study. Adv. Pharmacol. Sci. 2015, 2015, 164943. [Google Scholar] [CrossRef]
- Evans, M.; Elliott, J.G.; Sharma, P.; Berman, R.; Guthrie, N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: A multi-center, randomized, placebo-controlled study. Maturitas 2011, 68, 189–196. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, A.; Filice, F.; Gotti, S.; Panzica, G. Perinatal exposure to genistein affects the normal development of anxiety and aggressive behaviors and nitric oxide system in CD1 male mice. Physiol. Behav. 2014, 133, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Landa, J.F.; Hernández-Figueroa, J.D.; Hernández-Calderon, B.C.; Saavedra, M. Anxiolytic-like effect of phytoestrogen genistein in rats with long-term absence of ovarian hormones in the black and white model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 367–372. [Google Scholar]
- Rodríguez-Landa, J.F.; Hernández-López, F.; Saavedra, M. Involvement of Estrogen Receptors in the Anxiolytic-Like Effect of Phytoestrogen Genistein in Rats with 12-Weeks Postovariectomy. Pharmacol. Pharm. 2012, 3, 439–446. [Google Scholar]
- Wu, Z.M.; Ni, G.L.; Shao, A.M.; Cui, R. Genistein alleviates anxiety-like behaviors in post-traumatic stress disorder model through enhancing serotonergic transmission in the amygdala. Psychiatry Res. 2017, 255, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, S.; Qi, X.; Chen, S.; Chen, P.; Zhang, Q. Lose dose genistein inhibits glucocorticoid receptor and ischemic brain injury in female rats. Neurochem. Int. 2014, 65, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, J.; Shi, C.; Guo, K.; Yew, D.T. Effects of genistein on hippocampal neurodegeneration of ovariectomized rats. J. Mol. Neurosci. 2007, 31, 101–112. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, Q.H. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Br. J. Nutr. 2010, 104, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Hairi, H.A.; Shuid, A.N.; Ibrahim, N.; Jamal, J.A.; Mohamed, N.; Mohamed, I.N. The Effects and Action Mechanisms of Phytoestrogens on Vasomotor Symptoms During Menopausal Transition: Thermoregulatory Mechanism. Curr. Drug Targets 2019, 20, 192–200. [Google Scholar] [CrossRef]
- Campagnoli, C.; Abbà, C.; Ambroggio, S.; Lotano, M.R.; Peris, C. Differential effects of various progestogens on metabolic risk factors for breast cancer. Gynecol. Endocrinol. 2007, 23, 22–31. [Google Scholar] [CrossRef]
- Wachtel, M.S.; Yang, S.; Dissanaike, S.; Margenthaler, J.A. Hormone Replacement Therapy, Likely Neither Angel Nor Demon. PloS ONE 2015, 10, e0138556. [Google Scholar] [CrossRef]
- Cardenas-Trowers, O.; Meyer, I.; Markland, A.D.; Richter, H.E.; Addis, I. A Review of Phytoestrogens and Their Association With Pelvic Floor Conditions. Female Pelvic Med. Reconstr. Surg. 2018, 24, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Xu, W.; Zhang, Z. Analysis on the mechanism of Helicobacter pylori-induced apoptosis in gastric cancer cell line BGC-823. Int. J. Mol. Med. 2005, 16, 741–745. [Google Scholar] [PubMed]
- Reger, M.K.; Zollinger, T.W.; Liu, Z.; Jones, J.F.; Zhang, J. Dietary intake of isoflavones and coumestrol and the risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2018, 142, 719–728. [Google Scholar] [CrossRef] [PubMed]

| Symptoms/Disease | Genistein Effects |
|---|---|
| Vasomotor | Reduction of hot flashes, night sweats, and sleep disturbances frequency; as well as depression symptoms and memory loss |
| Cardiovascular | Reduction of myocardial necrosis, macrophage and serum levels of TNF-α, severity of atherosclerosis, and myocardial infarctions incidence |
| Obesity | Reduction of serum concentration of total cholesterol, LDL, triglycerides, and HDL |
| Diabetes | Reduction of fasting glucose concentration, insulin resistance, and improves glycemic metabolism |
| Cancer | Reduces the incidence of breast, hepatocellular, lung, gastric, and ovarian cancer |
| Stress responses | Improves 5-HT metabolism, stabilizes MAO activity, and improves turnover ratio of 5-HIAA/5-HT |
| Cancer | Cell Line | Genistein Concentration | Molecular Targets | Activity by Which Anti-Cancer Is Achieved | References |
|---|---|---|---|---|---|
| Breast | MCF-7 | 50 µM | NF-κB, AKT, BRCA1, BRCA2, HER2, EGFR, PDGFR, LRP, Abl | ↓ HER2 expression, apoptosis ↑ suppressor proteins | [69,70,71] |
| MDA-MB-231 | 30 µM | NF-κB, AKT, p21WAF1/CIP1, G1 Phase | ↓ phosphorylation of AKT and ↑ NF-κB DNA-binding activity, MDM-2-mediated degradation of p53, and p21WAFI | [72,73,74] | |
| Liver | HepG2 | 10–20 µM | TGF-β, NFAT1, FAK, EGFR, G2/M phase, NF-κB, MAPK, PI3K/AKT | Cell cycle arrest, ↓migration, MAPK, PI3K/AKT signaling pathways and apoptosis | [75] |
| Bel-7402 | 10 μg/mL | p125FAK, G0/G1 and G2/M phase | ↑ cell cycle arrest in the G0/G1 and G2/M phase, ↓ p125FAK | [75,76] | |
| HuH-7 | 20 µM | Caspase -3, -6, -7, -8, -10, MMP-9, NF-κB, MAPK/AP-1 and PI3K/AKT | ↑ apoptosis, fragmentation of DNA, ↓ NF-κB activity | [77,78] | |
| Hep3B | 15–25 µM | p38 MAPK, caspase, NF-κB, p53, AMPK | AMPK-mediated anti-inflammation and pro-apoptosis, ↓ TNF and IL-6, apoptosis, fragmentation of DNA, ↑ endoplasmic reticulum stress and mitochondrial insult | [76,78,79,80] | |
| SMMC-7721 | 10–20 µM | Caspase, NF-κB, G2/M phase, TGF-β, MAPK/AP-1, PI3K/AKT, p53 | Apoptosis, ↓ of NF-κB activity, ↑ cell cycle arrest in the G2/M phase | [81,82,83] | |
| Lung | A549 | 25–50 µM | EGFR, NF-κB, G2/M, miR-27a, MET and EGFR | Cell cycle arrest, apoptosis, G2 phase arrest, ↓MET protein expression levels, ↑ apoptosis via miR-27a and MET signaling pathways | [84,85,86] |
| H446 | 25 µM | FoXM1, Cdc25B, cyclin B, survivin | ↓ FoXM1, Cdc25B, cyclin B and survivin, ↑ apoptosis. | [81] | |
| Gastric | BGC-823 | 25 µM | Bcl-2, BAX, NF-κB, COX-2, G2/M, caspase-3, AKT | Apoptosis, ↓ Bcl-2, cell proliferation, G2/M Phase arrest, breakdown of caspases | [84,87,88] |
| SGC-7901 | 10–20 μg/mL | ERK1/2 (MAPK1/3) PI3K/AKT, PTEN, Ser642, Wee1, Cdc2/Cdk1, Thr15 | ↓tyrosine-specific protein kinases, phosphorylation of EP300 by inhibiting the activity of MAPK1, ↑ apoptosis | [89,90,91] | |
| Colon | DLD-1 cell line | 75 µM | Nuclear β-catenin, phospho-β-catenin, sFRP2, WNT pathway | ↓ β-catenin-mediated WNT signaling through increasing sFRP2 gene | [92] |
| SW480 | 10 µM | p21, cyclin D1, c-MYC, DKK1 | ↑ mRNA and DKK1 protein levels, ↓ cell proliferation, Induce histone acetylation | [93,94] | |
| HT29 | 60–120 µM | G2/M and S phases, p21WAF1, Bax/Bcl-2, phase, FOXO3 | G2/M phase cell cycle arrest, ↑ apoptosis through Bcl-2 family proteins, p21WAF1 during the cell cycle | [95,96] | |
| HCT116 | 25–50 µmol/L | Metalloproteinase, VEGF3, FOXO3, p53, PI3K/AKT, G2/M phase | Silencing of p53-determined activity of FOXO3, induce G2/M phase cell cycle arrest and apoptosis, ↓ MMP-2and Fms-related tyrosine Kinase 4. | [97,98] | |
| SW1116 | 10–30 µg/mL | G2/M Phase, PTKs, topoisomerase-II, PG/GAG | Cell cycle arrest in G2/M phase, ↓ protein tyrosine kinases and topoisomerase II, affects the synthesis of PG/GAG and ↓ cell proliferation | [99,100] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. https://doi.org/10.3390/molecules24213892
Thangavel P, Puga-Olguín A, Rodríguez-Landa JF, Zepeda RC. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules. 2019; 24(21):3892. https://doi.org/10.3390/molecules24213892
Chicago/Turabian StyleThangavel, Prakash, Abraham Puga-Olguín, Juan F. Rodríguez-Landa, and Rossana C. Zepeda. 2019. "Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases" Molecules 24, no. 21: 3892. https://doi.org/10.3390/molecules24213892