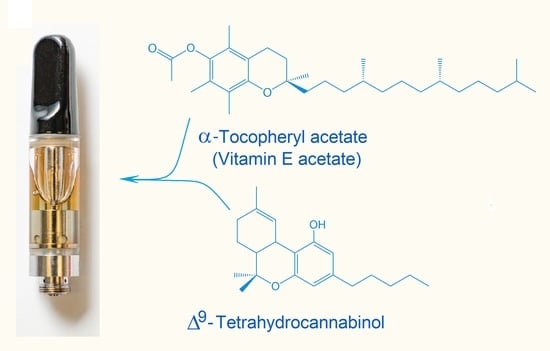
Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Procurement
2.2. Sample Processing
2.3. Analytical Standards and Reagents
2.4. GC-MS Untargeted Analysis
2.5. LC-HRMS/MS Untargeted Analysis
2.6. Pesticide Screening Using Orbitrap Mass Spectrometry
2.7. Quantitation of Cannabinoids
2.8. Analysis of Mycotoxins
2.9. Quantitative Analysis of Myclobutanil and PBO
2.10. Quantitative Analysis of VEA using GC-MS
2.11. Nuclear Magnetic Resonance (NMR) Spectroscopy
3. Results
3.1. Untargeted Analysis Identifies VEA
3.2. Targeted Analysis of Vaporizer Fluids
3.3. Pesticides and Mycotoxins
3.4. Analysis of Commercial Diluents and Thickeners
4. Discussion
4.1. Characterization of Illicit Cannabis Vaporizer Fluids
4.2. VEA and Other Diluents and Thickeners
4.3. Vaping and Pulmonary Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—Preliminary report. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Siegel, D.A.; Jatlaoui, T.C.; Koumans, E.H.; Kiernan, E.A.; Layer, M.; Cates, J.E.; Kimball, A.; Weissman, D.N.; Petersen, E.E.; Reagan-Steiner, S.; et al. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—United States, October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Davidson, K.; Brancato, A.; Heetderks, P.; Mansour, W.; Matheis, E.; Nario, M.; Rajagopalan, S.; Underhill, B.; Wininger, J.; Fox, D. Outbreak of electronic-cigarette-associated Acute Lipoid Pneumonia—North Carolina, July–August 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chow, W.; Wong, J.W.; Leung, D.; Chang, J.; Li, M. Non-target data acquisition for target analysis (nDATA) of 845 pesticide residues in fruits and vegetables using UHPLC/ESI Q-Orbitrap. Anal. Bioanal. Chem. 2019, 411, 1421–1431. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W.; Chang, J.; Wong, J.W. Development and validation of a qualitative method for target screening of 448 pesticide residues in fruits and vegetables using UHPLC/ESI Q-Orbitrap based on data independent acquisition and compound database. J. Agric. Food Chem. 2017, 65, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duffy, B.C.; Durocher, L.A.; Dittmar, M.A.; Acosta, R.A.; Delaney, E.R.; Li, L.; Aldous, K.M.; Spink, D.C. Potency analysis of medical marijuana products from New York State. Cannabis Cannabinoid Res. 2019, 4, 195–203. [Google Scholar] [CrossRef] [PubMed]
- New York State Department of Health—Wadsworth Center. Measurement of Phytocannabinoids in Medical Marijuana Using HPLC-PDA. NYS DOH MML-300; Revision 3; New York State Department of Health—Wadsworth Center: Albany, NY, USA, 2018. Available online: https://www.wadsworth.org/sites/default/files/WebDoc/NYS%20DOH%20MML-300-03.pdf (accessed on 14 September 2019).
- New York State Department of Health—Wadsworth Center. Measurement of Mycotoxins in Medical Marijuana by LC-MS/MS. NYS DOH MML-303-02; Revision 3; New York State Department of Health—Wadsworth Center: Albany, NY, USA, 2018. Available online: https://www.wadsworth.org/sites/default/files/WebDoc/NYS%20DOH%20MML-303-02.pdf (accessed on 14 September 2019).
- New York State Department of Health—Wadsworth Center. Determination of the Pesticide Myclobutanil and the Synergist Piperonyl Butoxide in Medical Marijuana Using LC-MS/MS with Positive Ion Detection. NYS DOH MML-307-02; Revision 4; New York State Department of Health—Wadsworth Center: Albany, NY, USA, 2018. Available online: https://www.wadsworth.org/sites/default/files/WebDoc/NYS%20DOH%20MML-307-02.pdf (accessed on 14 September 2019).
- Adams, A.J.; Banister, S.D.; Irizarry, L.; Trecki, J.; Schwartz, M.; Gerona, R. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 2017, 376, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.; Glass, M.; McGregor, I.S.; Connor, M.; et al. Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Boland, D.M.; Reidy, L.J.; Seither, J.M.; Radtke, J.M.; Lew, E.O. Forty-three fatalities involving the synthetic cannabinoid, 5-Fluoro-ADB: Forensic pathology and toxicology implications. J. Forensic. Sci. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, G.; Faggiani, F.; Bolchi, C.; Pallavicini, M.; Dei Cas, M. Ten years of fentanyl-like drugs: A technical-analytical review. Anal. Sci. 2019, 35, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. Current therapeutic cannabis controversies and clinical trial design issues. Front. Pharmacol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, G.R.B.; Sarna, L.P.; Mechoulam, R. Conversion of CBD to Δ8-THC and Δ9-THC. U.S. Patent 7,399,872, 15 July 2008. [Google Scholar]
- New York State Department of Health. New York State Department of Health Announces Update on Investigation into Vaping-Associated Pulmonary Illnesses. Available online: https://www.health.ny.gov/press/releases/2019/2019-09-05_vaping.htm (accessed on 5 September 2019).
- U.S. Food and Drug Administration. Lung Illnesses Associated with Use of Vaping Products. Available online: https://www.fda.gov/news-events/public-health-focus/lung-illnesses-associated-use-vaping-products#Analysis (accessed on 2 December 2019).
- Lewis, N.; McCaffrey, K.; Sage, K.; Cheng, C.-J.; Green, J.; Goldstein, L.; Campbell, H.; Ferrell, D.; Malan, N.; LaCross, N.; et al. E-cigarette use, or vaping, practices and characteristics among persons with associated lung injury—Utah, April–October 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Wiens, T.; Peterson, J.; Saravia, S.; Lunda, M.; Hanson, K.; Wogen, M.; D’Heilly, P.; Margetta, J.; Bye, M.; et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Downs, D. Vape Pen Lung Disease has Insiders Eyeing Misuse of New Additives. 2019. Available online: https://www.leafly.com/news/health/vape-pen-lung-disease-thc-oil-additive-investigation (accessed on 30 August 2019).
- Downs, D. Regulators, Industry React to Vitamin E-Tainted Vape Carts. 2019. Available online: https://www.leafly.com/news/industry/vapi-vape-pen-lung-disease-regulators (accessed on 14 September 2019).
- Downs, D. Vape Cart Additive Makers Pull Products as Others Go Dark. 2019. Available online: https://www.leafly.com/news/health/some-vape-cart-additive-makers-pull-products-others-go-dark (accessed on 14 September 2019).
- Dicpinigaitis, P.V.; Trachuk, P.; Fakier, F.; Teka, M.; Suhrland, M.J. Vaping-associated acute respiratory failure due to acute lipoid pneumonia. Lung 2019. [Google Scholar] [CrossRef] [PubMed]
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Butt, Y.M.; Smith, M.L.; Tazelaar, H.D.; Vaszar, L.T.; Swanson, K.L.; Cecchini, M.J.; Boland, J.M.; Bois, M.C.; Boyum, J.H.; Froemming, A.T.; et al. Pathology of vaping-associated lung injury. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mehrad, M.; Dammert, P.; Arrossi, A.V.; Sarda, R.; Brenner, D.S.; Maldonado, F.; Choi, H.; Ghobrial, M. Lung biopsy findings in severe pulmonary illness associated with e-cigarette use (vaping). Am. J. Clin. Pathol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [Green Version]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Pankow, J.F.; Talbot, P. High-nicotine electronic cigarette products: Toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem. Res. Toxicol. 2019, 32, 1058–1069. [Google Scholar] [CrossRef] [Green Version]
- Noël, J.C.; Ruzsanyi, V.; Rainer, M.; Bonn, G. Investigation of the evaporation behavior of aroma compounds in e-cigarettes. Anal. Bioanal. Chem. 2019, 411, 3029–3035. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Mishin, V.; Laskin, J.D.; Mainelis, G.; Wackowski, O.A.; Delnevo, C.; Schwander, S.; Khlystov, A.; Samburova, V.; Meng, Q. Hydroxyl radicals in e-cigarette vapor and e-vapor oxidative potentials under different vaping patterns. Chem. Res. Toxicol. 2019, 32, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical composition of aerosol from an e-cigarette: A quantitative comparison with cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Laughlen, G.F. Studies on pneumonia following naso-pharyngeal injections of oil. Am. J. Pathol. 1925, 1, 407–414. [Google Scholar] [PubMed]
- Cannon, P.R. The problem of lipoid pneumonia—A brief review. JAMA 1940, 115, 2176–2179. [Google Scholar] [CrossRef]
- Tancredi, A.; Graziano, P.; Scaramuzzi, R.; Scaramuzzi, G.; Carosi, I.; Attino, V.; Cuttitta, A.; Taurchini, M. Exogenous lipoid pneumonia due to chronic inhalation of oily product used as a lubricant of tracheotomy cannula. Eurasian J. Med. 2018, 50, 125–127. [Google Scholar] [CrossRef]
- Cha, S.; Choi, S.H.; Kim, H.J.; Kim, Y.J.; Lim, J.K.; Yoo, S.S.; Lee, S.Y.; Lee, J.; Kim, C.H.; Park, J.Y. Clinical and radiological manifestations of lipoid pneumonia according to etiology: Squalene, omega-3-acid ethyl esters, and idiopathic. Clin. Respir. J. 2019, 13, 328–337. [Google Scholar] [CrossRef]
- Marangu, D.; Pillay, K.; Banderker, E.; Gray, D.; Vanker, A.; Zampoli, M. Exogenous lipoid pneumonia: An important cause of interstitial lung disease in infants. Respirol. Case Rep. 2018, 6, e00356. [Google Scholar] [CrossRef]
- Marangu, D.; Gray, D.; Vanker, A.; Zampoli, M. Exogenous lipoid pneumonia in children: A systematic review. Paediatr. Respir. Rev. 2019. [Google Scholar] [CrossRef]
- Voulgareli, I.; Chronaiou, A.; Tsoukalas, D.; Tsoukalas, G. A rare case of lipoid pneumonia attributed to amiodarone. Pneumonia 2018, 10, 12. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Jiang, B. Exogenous lipid pneumonia in old people caused by aspiration: Two case reports and literature review. Resp. Med. Case Rep. 2019, 27. [Google Scholar] [CrossRef]
- Thanavala, Y.; Goniewicz, M.L. Vaping-induced severe respiratory disease outbreak: What went wrong? Lancet Resp. Med. 2019. [Google Scholar] [CrossRef]
- PubChem Compound Summary—Alpha-Tocopherol Acetate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Tocopherol-acetate#section=EPA-Safer-Chemical (accessed on 29 October 2019).
- Ushikusa, T.; Maruyama, T.; Niiya, I. Pyrolysis behavior and thermostability of tocopherols. J. Jpn. Oil Chem. Soc. 1991, 40, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wang, P.; Ito, K.; Fowles, J.; Shusterman, D.; Jaques, P.A.; Kumagai, K. Measurement of heating coil temperature for e-cigarettes with a "top-coil" clearomizer. PLoS ONE 2018, 13, e0195925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennick, K.A.; Warner, K. Effect of elevated temperature on development of tocopherolquinones in oils. J. Agric. Food Chem. 2006, 54, 2188–2192. [Google Scholar] [CrossRef]
- Kreps, F.; Burcova, Z.; Schmidt, S. Degradation of fatty acids and tocopherols to form tocopheryl quinone as risk factor during microwave heating, pan frying and deep-fat frying. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Merker, M.P.; Audi, S.H.; Bongard, R.D.; Lindemer, B.J.; Krenz, G.S. Influence of pulmonary arterial endothelial cells on quinone redox status: Effect of hyperoxia-induced NAD(P)H:quinone oxidoreductase 1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L607–L619. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Catalytic hydrogenation of squalene to squalane. Org. Process Res. Dev. 2014, 189, 1110–1115. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Morel-Espinosa, M.; Rees, J.; Sosnoff, C.; Cowan, E.; Gardner, M.; Wang, L.; Valentin-Blasini, L.; Silva, L.; et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use—Associated lung injury—10 states, August–October 2019. Morb. Mortal. Wkly. Rep. 2019. [Google Scholar] [CrossRef]





| Sample | Nicotine | CBG | CBD | THCV | CBN | Δ9-THC | Δ8-THC | THCA | CBC | VEA (%) | MCT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 2.13 | 0.91 | <0.884 * | <0.884 | 34.4 | <0.884 | <1.58 | <0.884 | 38.7 | - |
| 2 | - | 1.56 | <1.43 | <1.43 | <1.43 | 45.1 | <1.43 | <2.38 | <1.43 | 33.5 | - |
| 3 | - | 2.45 | 3.53 | <1.17 | <1.17 | 60.8 | <1.17 | <1.96 | <1.17 | <1.0 | + |
| 4 | + | <0.965 | <0.965 | <0.965 | <0.965 | <0.965 | <0.965 | <1.61 | <0.965 | <1.0 | - |
| 5 | - | 1.61 | <1.05 | <1.05 | 1.05 | 41.6 | <1.05 | <1.75 | <1.05 | 31.1 | - |
| 6 | - | <1.15 | 9.46 | <1.15 | <1.15 | 42.8 | <1.15 | <1.92 | <1.15 | 24.8 | - |
| 7 | - | <2.72 | <2.72 | <2.72 | <2.72 | 25.6 | <2.72 | <4.54 | <2.72 | 33.4 | + |
| 8 | - | <1.19 | 8.34 | <1.19 | <1.19 | 44.9 | <1.19 | <1.98 | <1.19 | 19.2 | - |
| 9 | - | <1.31 | 1.37 | <1.31 | <1.31 | 25.6 | <1.31 | <2.17 | <1.31 | 44.6 | - |
| 10 | - | <1.13 | <1.13 | <1.13 | <1.13 | 11.6 | 26.7 | 1.88 | <1.13 | 20.9 | - |
| 11 | - | <1.38 | <1.38 | <1.38 | <1.38 | 10.8 | 24.7 | <2.29 | <1.38 | 21.3 | - |
| 12 | - | 1.54 | 13.30 | <1.25 | 1.59 | 14.7 | <1.25 | <2.08 | <1.25 | 33.9 | - |
| 13 | - | 1.40 | <1.18 | <1.18 | <1.18 | 19.7 | <1.18 | 2.0 | <1.18 | 16.4 | - |
| 14 | - | <1.16 | <1.16 | <1.16 | <1.16 | 30.1 | <1.16 | <1.92 | <1.16 | 19.2 | + |
| 15 | - | 2.91 | <0.944 | <0.944 | <0.944 | 63.2 | <0.944 | <1.57 | 1.20 | <1.0 | + |
| 16 | + | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 | <2.50 | <1.50 | <1.0 | - |
| 17 | - | 1.49 | <1.17 | <1.17 | <1.17 | 39.2 | <1.17 | <1.95 | <1.17 | 30.4 | - |
| 18 | - | <1.18 | <1.18 | <1.18 | 4.80 | 3.11 | 47.1 | 9.1 | <1.18 | <1.0 | + |
| 19 | - | <1.21 | <1.21 | <1.21 | <1.21 | 23.2 | <1.21 | 2.1 | <1.21 | 47.4 | - |
| 20 | - | 1.65 | <1.34 | <1.34 | <1.34 | 33.3 | <1.34 | <2.24 | <1.34 | 38.6 | - |
| 21 | - | 1.61 | <1.14 | <1.14 | 1.37 | 23.2 | <1.14 | <1.90 | <1.14 | 39.6 | - |
| 22 | - | <1.17 | <1.17 | <1.17 | 1.67 | 18.0 | 11.3 | 4.0 | <1.17 | 57.6 | - |
| 23 | - | <1.16 | <1.16 | <1.16 | 1.52 | 11.8 | 12.3 | 4.1 | <1.16 | 51.5 | - |
| 24 | - | 2.81 | <1.15 | <1.15 | 4.21 | 32.1 | <1.15 | 4.3 | <1.15 | <1.0 | + |
| 25 | - | <1.21 | <1.21 | <1.21 | 1.90 | 32.7 | <1.21 | <2.01 | <1.21 | 39.8 | - |
| 26 | - | 5.31 | <1.14 | <1.14 | 5.11 | 32.9 | <1.14 | 3.6 | <1.14 | <1.0 | + |
| 27 | - | 2.15 | 5.72 | <1.14 | 2.84 | 47.2 | <1.14 | <1.91 | <1.14 | <1.0 | - |
| 28 | - | 2.38 | <1.13 | <1.13 | 4.24 | 40.4 | <1.13 | <1.88 | <1.13 | <1.0 | + |
| 29 | - | 3.54 | <1.58 | <1.58 | 4.62 | 20.6 | <1.58 | 7.7 | <1.58 | <1.0 | + |
| 30 | - | 4.69 | <1.14 | <1.14 | 4.40 | 28.3 | <1.14 | 3.1 | <1.14 | <1.0 | + |
| 31 | - | 2.65 | <1.14 | <1.14 | 3.55 | 66.3 | <1.14 | <1.89 | <1.14 | <1.0 | + |
| 32 | - | <1.22 | <1.22 | <1.22 | 4.28 | 4.80 | 38.1 | 4.4 | <1.22 | <1.0 | + |
| 33 | - | 2.14 | <1.12 | <1.12 | 6.86 | 34.5 | <1.12 | <1.86 | <1.12 | <1.0 | + |
| 34 | - | 2.23 | <1.26 | 2.29 | 4.03 | 54.7 | <1.26 | <2.11 | <1.26 | <1.0 | + |
| 35 | - | <1.16 | <1.16 | <1.16 | <1.16 | 29.1 | <1.16 | <1.93 | <1.16 | 51.2 | - |
| 36 | - | <1.10 | <1.10 | <1.10 | 1.65 | 12.1 | 12.9 | 3.9 | <1.10 | 43.5 | - |
| 37 | - | <1.19 | <1.19 | <1.19 | 1.59 | 16.8 | 11.7 | 4.2 | <1.19 | 38.4 | - |
| 38 | - | 1.95 | <1.29 | <1.29 | 1.70 | 27.1 | <1.29 | <2.15 | <1.29 | 57.1 | - |
| Sample | Screening for Synthetics | Mycotoxins (ng/g) | Myclobutanil (µg/g) | PBO (µg/g) | Other Pesticides Detected (>1 µg/g) | |
|---|---|---|---|---|---|---|
| Cannabinoids | Opioids | |||||
| 1 | neg * | neg | <4.8 ** | 1.97 | 1.38 | bifenazate, trans-permethrin, boscalid, bifentrin |
| 2 | neg | neg | <4.8 | 1.64 | 0.84 | bifenazate, diphenylamine, imidacloprid, cypermethrin, bifenthrin, fenpropathrin |
| 3 | neg | neg | <4.8 | <0.25 | 24.1 | trifloxystrobin, etoxazole, bifenthrin |
| 4 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. *** |
| 5 | neg | neg | <4.8 | 8.64 | <0.25 | N.D. |
| 6 | neg | neg | <4.8 | 0.42 | <0.25 | metalaxyl, etoxazole |
| 7 | neg | neg | <4.8 | 1.22 | 2.52 | cis-phenothrin |
| 8 | neg | neg | <4.8 | 3.04 | <0.25 | metalaxyl, etoxazole |
| 9 | neg | neg | <4.8 | <0.25 | <0.25 | trifloxystrobin |
| 10 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 11 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 12 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 13 | neg | neg | <4.8 | <0.25 | <0.25 | imidacloprid |
| 14 | neg | neg | <4.8 | 0.28 | 5.74 | bifenthrin, boscalid |
| 15 | neg | neg | <4.8 | 0.51 | 7.5 | tebuconazole, bifenazate |
| 16 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 17 | neg | neg | <4.8 | 0.51 | 0.53 | etoxazole, bifenthrin |
| 18 | neg | neg | <4.8 | 2.70 | <0.25 | bifenthrin |
| 19 | neg | neg | <4.8 | <0.25 | 2.72 | N.D. |
| 20 | neg | neg | <4.8 | 5.62 | 0.54 | N.D. |
| 21 | neg | neg | <4.8 | 1.02 | 1.54 | bifenazate |
| 22 | neg | neg | <4.8 | 3.53 | <0.25 | cis-phenothrin |
| 23 | neg | neg | <4.8 | <0.25 | <0.25 | cis-phenothrin |
| 24 | neg | neg | <4.8 | 2.12 | 1.80 | bifenthrin, trifloxystrobin, trans-permethrin, bifenazate |
| 25 | neg | neg | <4.8 | 6.51 | 10.8 | trifloxystrobin, pyrimethanil, methyl-kresoxim, trans-permethrin |
| 26 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 27 | neg | neg | <4.8 | 4.97 | 11.20 | bifenazate, bifentrin, trans-permethrin |
| 28 | neg | neg | <4.8 | <0.25 | <0.25 | trifloxystrobin, bifenazate |
| 29 | neg | neg | <4.8 | 1.99 | 1.40 | N.D. |
| 30 | neg | neg | <4.8 | <0.25 | <0.25 | bifenazate, diphenylamine, metalaxy, tebuconazole, cyprodinil, propamocarb, trifloxystrobin |
| 31 | neg | neg | <4.8 | 5.11 | 3.81 | bifenazate |
| 32 | neg | neg | <4.8 | 28.30 | <0.25 | N.D. |
| 33 | neg | neg | <4.8 | 1.96 | 2.32 | trifloxystrobin, bifenazate, bifenthrin |
| 34 | neg | neg | <4.8 | <0.25 | <0.25 | cyprodinil, buprofezin |
| 35 | neg | neg | <4.8 | 2.14 | 2.57 | N.D. |
| 36 | neg | neg | <4.8 | 1.05 | 1.05 | imidacloprid |
| 37 | neg | neg | <4.8 | <0.25 | <0.25 | N.D. |
| 38 | neg | neg | <4.8 | <0.25 | I.M. **** | bifenazate |
| Product | Components |
|---|---|
| Diluent 1 | VEA |
| Diluent 2 | VEA |
| Diluent 3 | Squalane, MCT oil, triethyl citrate (minor) |
| Thickener 1 | VEA |
| Thickener 2 | α-Bisabolol, isophytol (minor) |
| Thickener 3 | Squalane |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duffy, B.; Li, L.; Lu, S.; Durocher, L.; Dittmar, M.; Delaney-Baldwin, E.; Panawennage, D.; LeMaster, D.; Navarette, K.; Spink, D. Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics 2020, 8, 8. https://doi.org/10.3390/toxics8010008
Duffy B, Li L, Lu S, Durocher L, Dittmar M, Delaney-Baldwin E, Panawennage D, LeMaster D, Navarette K, Spink D. Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics. 2020; 8(1):8. https://doi.org/10.3390/toxics8010008
Chicago/Turabian StyleDuffy, Bryan, Lingyun Li, Shijun Lu, Lorie Durocher, Mark Dittmar, Emily Delaney-Baldwin, Deepika Panawennage, David LeMaster, Kristen Navarette, and David Spink. 2020. "Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent" Toxics 8, no. 1: 8. https://doi.org/10.3390/toxics8010008