Abstract
Attributions are constantly assigned in everyday life. A well-known phenomenon is the self-serving bias: that is, people’s tendency to attribute positive events to internal causes (themselves) and negative events to external causes (other persons/circumstances). Here, we investigated the neural correlates of the cognitive processes implicated in self-serving attributions using social situations that differed in their emotional saliences. We administered an attributional bias task during fMRI scanning in a large sample of healthy subjects (n = 71). Eighty sentences describing positive or negative social situations were presented, and subjects decided via buttonpress whether the situation had been caused by themselves or by the other person involved. Comparing positive with negative sentences revealed activations of the bilateral posterior cingulate cortex (PCC). Self-attribution correlated with activation of the posterior portion of the precuneus. However, self-attributed positive versus negative sentences showed activation of the anterior portion of the precuneus, and self-attributed negative versus positive sentences demonstrated activation of the bilateral insular cortex. All significant activations were reported with a statistical threshold of p ≤ .001, uncorrected. In addition, a comparison of our fMRI task with data from the Internal, Personal and Situational Attributions Questionnaire, Revised German Version, demonstrated convergent validity. Our findings suggest that the precuneus and the PCC are involved in the evaluation of social events with particular regional specificities: The PCC is activated during emotional evaluation, the posterior precuneus during attributional evaluation, and the anterior precuneus during self-serving processes. Furthermore, we assume that insula activation is a correlate of awareness of personal agency in negative situations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
People evaluate their own and others’ behavior by seeking (“attributing”) causes for the occurrence of social events. The cognitive and emotional processes involved in these ascriptions are the focus of neuroscientific research on attributional patterns, which is based on the assumptions of attribution theory. Research in this field is diverse, and the findings are inconsistent. Therefore, the main goal of our study was to extend the knowledge about self-attribution processes by giving a detailed overview of the current and classical literature, using a large sample size in a study of the neural correlates of self-attribution processes, and providing a careful analysis and interpretation of our results.
The origins of attribution theory date back to the 1940s and 1950s, when Heider authored his seminal treatises “Social Perception and Phenomenal Causality” (1944) and The Psychology of Interpersonal Relations (1958). Heider divided people’s explanations (“attributions”) for the occurrence of different events into two types of causes: personal and environmental. Subsequent enhancements, systematizations, and reinterpretations of attribution theory were elaborated by Kelley (1967), Jones and Davis (1966), and later by Weiner (1974, 1986). Today numerous models have been proposed to explain attributional processes, which can be summarized under the generic term attribution theory.
In this sense, Försterling (2001) defined attribution theory as a group of theories on “how common sense operates” (p. 3), focusing on “the processes that make our everyday circumstances understandable, predictable, and controllable,” and which findings are “applicable to a wide area of domains such as achievement, love, health, friendship, and pathology” (p. 4). Attribution theory is a cognitive approach in psychology, and thus the research on attribution focuses on thoughts or cognitions concerning “how individuals select, process, store, recall, and evaluate (causally relevant) information and how the information is then used to draw causal inferences” (Försterling, 2001, p. 10).
Research in the field of attribution theory has revealed that attributions are susceptible to errors and biases (Försterling, 2001), such as attribution errors (Ross, 1977), attribution asymmetry (Frieze & Weiner, 1971), or the self-serving bias (Hastorf, Schneider, & Polefka, 1970). The so-called attributional bias is an umbrella term for different psychological phenomena that underlie the process of attributing responsibility/causation for various events or actions to different causes. Research on the phenomena of attributional biases has a long tradition in social psychology. These have been investigated in different ways: in terms of ego defense and the need for control (Cialdini, Braver, & Lewis, 1974; Kelley, 1967, 1987; Luginbuhl, Crowe, & Kahan, 1975), actor–observer differences (Jones & Nisbett, 1987; Mischel, 1968; Ross, 1977), primacy effects (Kanouse, 1971), and responsibility for accidents (Vidmar & Crinklaw, 1974; Walster, 1966, 1967) (for a detailed summary, see Fischhoff, 1976). Heider (1958) remarked that the process of attribution is influenced by the personal needs, feelings, and emotions of the one who attributes. Jones and Davis (1966) took a closer look at this personal involvement as a component of the attribution process and considered two manifestations: hedonic relevance and the variable of personalism (Shaver, 1975). Kelley (1967) expanded this area of study and distinguished between self-attributions and attributions to others, as well as the environmental context. This has led to different understandings of attributional biases. One aspect is the self-serving bias (SSB). The basic assumption here is that people tend to attribute events with positive outcomes to internal causes (themselves) and events with negative outcomes to external causes (the other person[s] involved or the situation; Hastorf et al., 1970; Kelley, 1973; for a summary, see Zuckerman, 1979). Hewstone (1989) pointed out two components of SSB: the “self-enhancing bias” (a tendency to attribute success rather to internal causes) and the “self-protecting bias” (a tendency to attribute failure rather to external causes). In the context of a certain blurring of concepts in current research, it is important to note at this point—as Hewstone did—that the research about SSB involves common-sense explanations and that the attribution of causes to persons in this context is not a legal, moral, or philosophical issue. Thus, it has to be distinguished clearly from the concepts of blame, cause, and responsibility (Hewstone, 1989).
More recently, several neuroscientific studies have investigated the neural correlates of the “attributional bias” using different techniques. Blackwood et al. (2003), Harris, Todorov, and Fiske (2005), and Seidel et al. (2010) employed functional magnetic resonance imaging (fMRI), and Krusemark, Campbell, and Clementz (2008) used electroencephalography. Blackwood et al. examined the neural correlates of the self-serving bias with an attributional decision task analogous to the Internal, Personal, and Situational Attributions Questionnaire (IPSAQ; Kinderman & Bentall, 1996). Their subjects had to decide whether ten positive and ten negative statements describing social situations taken from the IPSAQ were caused by themselves, by other persons involved, or by the situation. The authors found that self-responsibility attributions, in contrast to personal and situational attributions, were related to activations in the left lateral cerebellar hemisphere (lobule V), bilateral dorsal premotor cortex, and right lingual gyrus. Self-serving attributions were related to bilateral caudate nucleus activations, whereas non-self-serving attributions (i.e., external attribution of positive events and internal attribution of negative events) were associated with activations in the left lateral orbitofrontal cortex, right angular gyrus (AG), and right middle temporal gyrus (mTG). The authors concluded that the self-serving bias is mediated by the dorsal striatum, which as well is implicated in motivated behavior. Furthermore, they suggested that self-responsibility, as being social cognition of a higher order, is related to simpler internal models of goal-directed action, as reflected by activation of the bilateral premotor cortex and the cerebellum (regions substantially implicated in action simulation; Blackwood et al., 2003). Harris et al. used an attribution paradigm in which subjects had to make an attributional decision about the causes of several social events after getting additional information about the consensus, distinctiveness, and consistency of the described event. The authors found dispositional attributions (i.e., attributions of perceived behavior to the internal states of persons, such as unique attitudes, individual personality, or idiosyncratic intent) to be related to activations mainly in the right superior temporal sulcus (STS), the bilateral medial prefrontal cortex (MPFC), the right mTG, and the right precuneus. They concluded that common regions underlie social cognition—such as in theory-of-mind and attribution tasks—and that specific neural activation patterns are associated with unique dispositional attributions (Harris et al., 2005). An event-related potential study from Krusemark et al. used a facial working memory task in which their subjects got false (success or failure) feedback. Non-self-serving attributions were associated with activity in the left MPFC. Together with the results from previous studies (Amodio & Frith, 2006; Ochsner et al., 2004; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004), these results were interpreted as indicating that unbiased attributions require greater self-control (Krusemark et al., 2008). Seidel et al. (2010) used a social-situation paradigm to analyze the neural correlates of internal and external attributions in social events and of the self-serving bias. Their subjects had to decide whether the presented positive and negative social situations were caused mainly by themselves, the other person involved, or the situation itself. A contrast between internal and external attributions revealed activations along the right temporo-parietal junction (TPJ), in the right supramarginal gyrus, and in the superior temporal gyrus (STG) bilaterally. The reverse contrast (external vs. internal attributions) revealed activations of the left parieto-frontal network involving the lateral and medial parietal areas and the superior frontal regions, with activations in the left and right precuneus, in the right cuneus, and along the left TPJ, including activations in the left AG, left mTG, and the left superior, middle, and superior medial frontal gyrus. Bilateral dorsal anterior cingulate cortex (ACC) activation correlated with the self-serving bias. These results support the assumption that a fronto-temporo-parietal network is involved in differentiating self and external responsibility, whereas the correlation of activations in the dorsal ACC and dorsal striatum with the self-serving bias was understood as being linked to the bias’s rewarding value (Seidel et al., 2010).
Additionally, further studies using different tasks have examined these same underlying cognitive phenomena. For instance, Moran, Macrae, Heatherton, Wyland, and Kelley (2006) used fMRI to investigate the dissociation between cognitive and affective components in self-reflective processes. These authors provided a task in which subjects had to judge the self-descriptiveness of favorable and unfavorable trait words. They reported that activity in the MPFC and PCC differed in context of increasing self-relevance, regardless the valence of the stimuli, whereas the activity of the ventral ACC was dependent on valence.
Beer and Hughes (2010) investigated the neural correlates of the “above-average” effect—which refers to a self-evaluation bias—with a modified version of a social comparative task. The authors reported that activity in the MPFC and the PCC correlated with reduced susceptibility to “above-average” judgments and activity of the ventral ACC correlated with differentiation of positive from negative valences, whereas activity in the orbitofrontal cortex (OFC) and the dorsal ACC correlated negatively with the “above-average” effect.
Finally, Hughes and Beer (2012) examined in their fMRI study “whether neural regions previously implicated in self-serving cognition relate to changes in decision thresholds underlying the extent to which judgments are self-serving” (Hughes & Beer, 2012, p. 890). By providing a modified version of the Over-Claiming Questionnaire and an accountability manipulation, the authors could show a correlation between activation in the OFC, the MPFC, and the dorsal ACC and a reduced self-serving bias. Moreover, the less pronounced was the self-serving bias, the more correlated was activity in the medial OFC.
In summary, although several imaging studies have employed very similar tasks (especially Blackwood et al., 2003, and Seidel et al., 2010), they have reported very different results. This could be due to the fact that imaging studies on “attributions in social situations” hitherto have operated only with relatively small sample sizes (e.g., Blackwood et al., 2003: n = 12). Accordingly, in our study we aimed to increase the statistical power used to investigate the neural basis of self-attribution in positive and negative social events by running a high number of subjects, and to validate the results with the original Internal, Personal and Situational Attributions Questionnaire, Revised German Version (IPSAQ-R).
To overcome some of the problems of single studies with small sample sizes, Sperduti, Delaveau, Fossati, and Nadel (2011) conducted a quantitative meta-analysis across 15 positron emission tomography and fMRI social cognition studies examining the neural correlates of internal and external agency attributions, in order to cluster (in)consistent findings. The authors detected—among other activations—two brain regions that were consistently implicated: the precuneus and the insula. The precuneus is involved in social processes such as perspective taking (Ruby & Decety, 2001, 2003), observation of social interactions (Iacoboni et al., 2004), self-referential processes (Iacoboni et al., 2004; Kircher et al., 2000; Spreng, Mar, & Kim, 2009; van der Meer, Costafreda, Aleman, & David, 2010), and causal attribution of social events (Seidel et al., 2010). In addition, Sajonz et al. (2010) discussed an anterior–posterior differentiation of the precuneus, with the anterior portion being more involved in self-referential processes, and the posterior portion being more linked to episodic memory processes. Furthermore, the precuneus has been discussed, along with the MPFC, as being involved in self-referential processes (Gusnard, Akbudak, Shulman, & Raichle, 2001; Kircher et al., 2002; Kircher et al., 2000; Ruby & Decety, 2003; for a review, see Schilbach, Bzdok, et al., 2012) and external attribution (Seidel et al., 2010; Sperduti et al., 2011), or in both processes (Ruby & Decety, 2001). Insula activation has been linked to self- and current-state-related phenomena related to physiological and emotional awareness and consciousness (Craig, 2009, 2010; Lamm & Singer, 2010; Singer, Critchley, & Preuschoff, 2009), as well as to self-agency attribution (e.g., Farrer & Frith, 2002; Leube et al., 2003; Sperduti et al., 2011).
In summary, a wide variety of brain regions have been linked to attributional biases, mostly including the precuneus and the insula, but with partly inconsistent findings across various studies. In our own work, we tested a large sample of healthy subjects with fMRI in order to investigate the neural correlates of the cognitive processes involved in internal and self-serving attributions across different positively or negatively valenced social situations. We used the IPSAQ scale as a basis for our fMRI task, but changed the context and semantics of the statements in order to create novel social events with higher ecological validity, that were more closely related to real life (Schilbach, Timmermans et al., in press). Furthermore, we compared our behavioral results from the fMRI task with results from the German version of the original “paper-and-pencil” IPSAQ-R (Moritz et al., 2010) in the same subjects, so as to test the reliability and validity of our paradigm. With regard to self-attribution, we expected to find activation in a fronto-temporo-parietal network (Blackwood et al., 2003; Seidel et al., 2010), as components of this network have been discussed in the context of self-processing (D’Argembeau et al., 2005; Farrer et al., 2003; Johnson et al., 2002; Vogeley et al., 2001). Furthermore, since we included a very large sample size, and therefore attained sufficient statistical power, we hypothesized that we would find a precise distinction among the different subregions of the posterior medial parietal cortex (precuneus) that are involved in differential self-attributional processes (for a review, see Cavanna & Trimble, 2006).
Method
Subjects
A group of 89 healthy subjects from the POSITIVE Study, a randomized-controlled multicenter clinical trial (Klingberg et al., 2010), were recruited at six German universities (measurements took place at five of the study sites). The inclusion criteria were (1) age in the range from 18 to 59 years; (2) the absence of neurorological or other medical conditions that could affect the results; (3) no mental disorder, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), in either the past or present, as assessed with the Structured Clinical Interview for the DSM-IV, German version (SCID-I; Wittchen, Wunderlich, Gruschwitz, & Zaudig, 1997); (4) right-handedness (as tested with the Edinburgh Handedness Inventory; Oldfield, 1971); and being (5) a native German speaker with (6) normal or corrected-to-normal vision and (7) no criteria for MRI exclusion (such as metal implants, pregnancy, etc.).
Eighteen subjects had to be excluded from the analysis: The fMRI data from eight subjects did not meet the quality criteria (in seven of the subjects the images did not cover the whole brain, and one had scanner-related artifacts; see Stöcker et al., 2005), eight further subjects were excluded due to technical problems (four incomplete data sets, two with intolerable repetition time [TR] deviations of more than 0.1 s, one with a wrong response-button configuration, and one missed trigger pulse), and two were excluded for not following the instructions properly. Thus, 71 subjects (34 females, 37 males) were finally included in the analyses reported here.
The 71 subjects had a mean age of 34.39 years (SD = 9.06) and a mean estimated verbal intelligence quotient of 115.12 (SD = 17.36) according to the German multiple-choice vocabulary test (MWT-B; Lehrl, 2005). After a complete description of the procedure, the subjects provided written informed consent to participate in the study. We received approval for the study from the local ethics committees at all sites, and carried it out in accordance with the latest version of the Declaration of Helsinki. After subjects had provided consent, the cognitive tests and the fMRI experiment were carried out. Because of a further application in a pre-/posttherapy study, a neuropsychological battery consisting of tests measuring verbal intelligence, attention, executive functions, and memory, as well as the IPSAQ-R (Moritz et al., 2010) and the beads-in-a-jar task (Garety, Hemsley, & Wessely, 1991), were administered in an adapted computer version (Moritz & Lincoln, 2008). Finally, the subjects were paid for their participation.
Tasks and stimuli
fMRI attributional bias task
For the fMRI attributional bias task, we used statements consisting of one sentence, related to those of the IPSAQ. However, the number of sentences was increased to 80 (40 with positive and 40 with negative connotations). We also modified their content: Instead of referring to “a friend” or “a neighbor,” as in the original IPSAQ, we expanded the other person involved to different identities that were implicated in different social situations. The final 40 positive (e.g., “Your boss appreciates your work in the team”) and 40 negative (e.g., “The waitress ignores you in the bar”) social situations consisted of six- to eight-word sentences, which all had the same syntactic structure: subject (singular), predicate (present tense), and direct object (the person him- or herself, or another person), followed by an indirect object, or a prepositional phrase.
Due to its further application in a pre-/posttherapy study reported elsewhere, two versions of the paradigm were created with two different sets of statements. In a pretest, both versions were each presented to 13 healthy subjects, who were asked to evaluate them according to criteria for (1) positivity/negativity, (2) plausibility, (3) emotionality, and (4) conceivability, each on a six-point scale. The positive and negative statements did not differ (Wilcoxon test for paired samples) with regard to plausibility (positive sentences, median [x med] = 3.98; negative sentences, x med = 3.84; Z = –1.15, p = .249), emotionality (positive sentences, x med = 3.53; negative sentences, x med = 3.83; Z = –0.73, p = .463), or conceivability (positive sentences, x med = 3.83; negative sentences, x med = 3.85; Z = –0.70, p = .484). However, as intended, the positive and negative statements clearly differed with regard to positivity (x med = 4.71 vs. 1.13, respectively; Z = –3.18, p = .001) and negativity (x med = 1.15 vs. 4.43, respectively; Z = –3.18, p = .001). The positive and negative sentence were presented in a pseudorandomized order. The two paradigm versions were randomly distributed across subjects.
During the fMRI experiment, the sentences were presented visually in white letters on a black screen, in a miniblock design, for 5 s each. Every statement was followed by a jittered interstimulus interval (3.25–10.75 s) during which a white fixation cross appeared on the black screen. The subjects were asked to read the sentences, to imagine the situation happening to them vividly, and to decide about the main cause of the situation by answering the question “Who has caused the situation?” Subjects had to indicate their attributional decisions about whether the situations had been caused by themselves or by the other person involved (i.e., internal vs. external cause) by a buttonpress with either the right index or middle finger, respectively. The correct use of the button box was checked before the experiment started. For a schematic representation of the experimental setup, see Fig. 1.
Behavioral task outside the scanner: IPSAQ-R
The IPSAQ-R, validated in German (Moritz et al., 2010), is a translated version of the original IPSAQ (Kinderman & Bentall, 1996) consisting of 16 items describing eight positive and eight negative situations. For each item, subjects are asked to put themselves in the position of someone experiencing a particular situation, to infer the most probable causal explanation for the situation, and to write down this explanation. Then they are asked to estimate (as a percentage) whether their causal explanations are due to internal, personal, or situational factors. Three positive interdependent and three negative interdependent subscales are calculated by adding up the percent ratings of internal, personal, and situational attributions for the positive and negative events. Additionally, an externalizing bias is computed by subtracting the internal negative score from the internal positive score, and a personalizing bias is calculated by dividing the personal negative score by the sum of the personal negative score and the situational negative score. Moreover, a monocausality score counts the number of items rated in a monocausal way (i.e., attributional scores that are rated with a minimum score of 90 %). The IPSAQ-R was administered to the subjects within two weeks prior to the fMRI experiment. As described above, the statements of the IPSAQ-R and the fMRI attributional bias task differed in their number and content (cf. the previous section).
fMRI data
Acquisition
We conducted fMRI measurements at five study sites (Bonn, Düsseldorf, Frankfurt am Main, Jülich, and Tübingen), all using 3-T Tim Trio magnetic resonance scanners (Siemens Medical Systems). Functional images were collected using echoplanar imaging sensitive to blood oxygenation level dependent (BOLD) contrast (T2*, 64 × 64 matrix, FoV 200 × 200 mm, voxel size = 3.1 × 3.1 × 3 mm, 36 slices, gap = 10 %, 3 mm thickness, TR = 2.25 s, TE = 30 ms, flip angle = 90º). The slices were measured in ascending order, were positioned transaxially parallel to the anterior–posterior commissural line (AC–PC), and covered the whole brain. A total of 360 functional images were collected. The initial three images were excluded from further analysis in order to remove the influence of T1 stabilization effects.
Quality control
The fMRI multicenter study was carefully planned and monitored. A detailed study protocol was developed in order to obtain homogeneous data. The sequences, the paradigm, and the comparability of the scanners were evaluated before the start. Therefore, reliability measurements were performed with 13 healthy subjects who were scanned by means of three identical paradigms (simple visual and motor tasks different from the ones reported here) at all of the fMRI centers involved. Furthermore, the MRI sequences and sequence comparability were evaluated across the entire data-acquisition phase by means of MRI phantom measurements in which we applied identical functional sequences on each scanning day. The Percent Signal Fluctuation index (PSF4) was calculated for the phantom data so as to constantly evaluate and control for center- or scanner-specific signal fluctuation/noise. Data acquisition was further monitored and supervised by means of monthly telephone conferences and several site visits. Finally, we used a fully automated quality assurance routine for fMRI (Stöcker et al., 2005) and discarded poor fMRI data (see the Subjects section).
Analysis
Preprocessing and first- and second-level analyses of the functional data were performed using the Statistical Parametric Mapping software (SPM5; www.fil.ion.uc.ac.uk/spm). All of the functional images were slice-time corrected, realigned, and resliced to the first image in order to correct for interscan movement; normalized to the standard Montreal Neurological Institute (MNI) template in SPM (with a resulting voxel size of 2 × 2 × 2 mm); smoothed (8-mm full-width-at-half-maximum isotropic Gaussian filter); and high-pass filtered (cutoff period 128 s).
Our statistical analysis was performed in a two-level procedure. At the first level, the BOLD responses for the presentation of the positive and negative sentences were modeled in miniblocks (duration = 5 s) convolved with the canonical hemodynamic response function. Movement parameters and buttonpresses were included in the model as regressors of no interest. The effects for the positive and negative sentences were added as contrasts and estimated using the general linear model approach. Because of variability in the design efficiency caused by imbalanced responses, on the first level, we modeled solely the positive and negative sentences with two regressors, and abandoned the option to estimate additional contrasts for internal and external attributions, since—consistent with the hypothesis of a self-serving bias—several subjects did not have enough buttonpresses for each condition, and therefore not enough events for the statistical analysis (e.g., 36 of the subjects had fewer than ten “self” buttonpresses for the negative sentences). Thus, in contrast to Blackwood et al.’s (2003) approach, we had no need to exclude any subject due to missing trials per condition, because we incorporated the attributional decision as a covariate of interest at the second level (see the following description).
The single-subject β contrasts relating to positive and negative sentences were used for further analyses. On the second level, a paired t test was calculated for the whole sample of the 71 included subjects in order to investigate the neural correlates of internal and external attributions in positive or negative situations. Statistical parametric maps were computed for the contrasts Positive Sentences > Negative Sentences and Negative Sentences > Positive Sentences.
Additionally, internal and external attributions were modeled as a covariate of interest containing only the internal/external ratio (i.e., the percentage of “self” buttonpresses, with higher values indicating more self-attributions), and not the single numbers of internal and external attributions. This covariate interacted with the experimental factor Emotional Valence, and was therefore split into two parts. Thus, we could estimate different statistical parametric maps by weighting the split covariate and had enough degrees of freedom (T66) to calculate the following contrasts. With this setup, we addressed three psychological domains (emotion, self-attribution, and biased self-attribution) that underlie attributional evaluation processes, utilizing the following contrasts.
-
1.
Emotion: Positive Situations > Negative Situations and Negative Situations > Positive Situations. These contrasts refer to emotional evaluations of the situations.
-
2.
Self-attribution: Internal Attribution (i.e., more self- than other-attributions in both types of situations). This contrast refers to self-attribution processes, regardless of the emotional contents of the situations.
-
3.
Biased self-attribution: Internal Attribution Positive > Internal Attribution Negative (i.e., more self-attributions in positive situations > more self-attributions in negative situations) and Internal Attribution Negative > Internal Attribution Positive (i.e., more self-attributions in negative situations > more self-attributions in positive situations). These contrasts refer to the phenomenon of biased self-attribution processes that depend on the emotional contents of the situations (Moritz et al., 2010).
In addition, we estimated further statistical parametric maps for exploratory analyses: External Attribution (i.e., more other- than self-attributions in both types of situations), Internal Attribution Positive (i.e., more self- than other-attributions in positive situations), Internal Attribution Negative (i.e., more self- than other-attributions in negative situations), External Attribution Positive (i.e., more other- than self-attributions in positive situations), and External Attribution Negative (i.e., more other- than self-attributions in negative situations).
The covariates of no interest were buttonpresses, modeled by the mean reaction times of each subject; Misses, containing the individual numbers of omitted buttonpresses; and Recruiting Center, consisting of the six sites (coded as five dummy regressors) from which the subjects were recruited.
Significant activations are reported for a statistical threshold of p ≤ .001, uncorrected, with a cluster extent threshold of k ≥ 20 voxels. Brain activation was plotted on the anatomical SPM template. Anatomical localizations of significant activation in local maxima of the MNI coordinates were identified by using the SPM Anatomy Toolbox (Eickhoff et al., 2005; www.fz-juelich.de/ime/spm_anatomy_toolbox).
Behavioral and neurocognitive data analysis
Statistical analyses were performed using SPSS 20. With regard to the fMRI attributional bias task, the ratios of internal attributions for positive and negative events were computed controlling for missing data. The number of internal attributions for either positive or negative events was divided by the total number of these events that subjects responded to.
With regard to the IPSAQ-R, six of the subjects had to be excluded from the analyses for answering fewer than 75 % of the questions (n = 4) or for not following the instructions properly (n = 2). The percentage ratings were transformed so that their sum equaled 100 % (Moritz et al., 2010). The ratios of internal, personal, or situational attributions for positive and negative events were divided by the number of events that subjects responded to.
In order to test for convergent validity, bivariate relations between the behavioral responses during the fMRI attributional bias task and the IPSAQ-R were computed with Spearman correlation coefficients and corrected for multiple comparisons using a Bonferroni correction.
Results
Behavioral data
fMRI attributional bias task
Table 1 depicts the means and standard deviations of the behavioral responses during the fMRI paradigm. As expected, on a descriptive level the subjects showed more internal attributions (attributions to oneself) for positive than for negative events and a self-serving bias (number of internal attributions for positive events minus number of internal attributions for negative events). Of our subjects, 37 showed a self-serving bias, only nine showed a non-self-serving bias, and 25 showed neither of the two biases. The reaction times did not differ significantly in any of the conditions. The mean reaction times were 3.18 s (SD = 0.84) for internal attributions in positive sentences, 3.18 s (SD = 0.76) for external attributions in positive sentences, 3.44 s (SD = 0.78) for internal attributions in negative sentences, and 3.07 s (SD = 0.75) for external attributions in negative sentences.
IPSAQ-R results
Table 2 depicts the means and standard deviations from the behavioral IPSAQ-R, assessed outside of the scanner. On a descriptive level, the subjects showed mostly internal attributions for positive events, whereas negative events were attributed primarily to personal causes (the other person involved). Furthermore, an externalizing bias and a personalizing bias were present.
Validation of the IPSAQ-R and the fMRI attributional bias task
For a comparison of the percentage ratios of the fMRI attributional bias task and the IPSAQ-R, see Fig. 2. The convergent validity of the fMRI task was investigated using Spearman correlation analyses. The following positive correlations between the fMRI task and the IPSAQ-R were found: Numbers of internal attributions for positive sentences (r = .35, p = .004), numbers of personal attributions for positive sentences (r = .39, p ≤ .001), numbers of personal attributions for negative sentences (r = .30, p = .016), and self-serving bias in both tasks (r = .28, p = .023) were associated with each other, whereas the internal attributions for negative events (r = .09, p = .456) were uncorrelated.
Depiction of percentage ratios (means and standard deviations) from internal and external attributions in the fMRI attributional bias task (dark gray) and the German revived version of the Internal, Personal, and Situational Attributions Questionnaire (IPSAQ-R; Moritz et al., 2010) (light gray)
fMRI data
To cluster our findings with regard to the neural correlates of cognitive processes involved in internal and self-serving attributions across the positively and negatively valenced social situations, we divided our results into three types of whole-brain contrasts by addressing the three psychological domains involved (emotion, self-attribution, and biased self-attribution). Furthermore, we report the results of our exploratory analysis.
Emotion
The Positive Sentences > Negative Sentences contrast revealed activations of the angular gyrus bilaterally (BA 7/39), the right posterior cingulate cortex (BA 23), the right superior frontal gyrus (BA 8), the middle frontal gyrus bilaterally (BA 6/8/45), the left superior frontal gyrus (BA 6/8), and the right inferior temporal gyrus (BA 20). Negative Sentences > Positive Sentences resulted in activation of the left middle temporal gyrus bilaterally (BA 20/22), the left lingual gyrus (BA 17/18), the calcarine gyrus bilaterally (BA 17/18), the left cuneus (BA 17), the left posterior cingulate cortex (BA 4), the supramarginal gyrus bilaterally (BA 42/48), the right superior temporal gyrus (BA 21), the right angular gyrus (BA 48), and the left middle occipital gyrus (BA 39) (Table 3).
Self-attribution
Internal Attribution revealed activation only of the right precuneus (BA 7) (Table 3).
Biased self-attribution
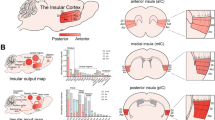
Internal Attribution Positive > Internal Attribution Negative revealed activation of the right precuneus (BA 7), whereas Internal Attribution Negative > Internal Attribution Positive resulted in activation of the insular lobe bilaterally (BA 45/47) (see Fig. 3), the left inferior frontal gyrus [pars triangularis (BA 44/45) and pars orbitalis (BA 47)], and the middle frontal gyrus bilaterally (BA 44/46) (Table 3).
For a schematic depiction of the activations of the posterior cingulate cortex and the precuneus described above in the three conditions (emotion, self-attribution, and biased self-attribution), see Fig. 4.
Exploratory analysis
Internal Attribution Positive revealed activation of the right precuneus (BA 7), whereas Internal Attribution Negative resulted in activation of the left postcentral gyrus (BA 4) and the left supplementary motor area (BA 6/32). External Attribution showed no significant results. External Attribution Positive revealed activation of the left middle frontal gyrus (BA 46) and the left inferior frontal gyrus (BA 45), whereas External Attribution Negative showed no significant results (Table 3).
Discussion
In this study, we investigated a large sample of healthy subjects with fMRI to explore the neural correlates of the cognitive processes implicated in self-attributions of positively and negatively valenced situations. We have validated our fMRI task by means of the original paper-and-pencil version of the IPSAQ-R (Moritz et al., 2010).
Contrary to most of the previous findings in neuroscientific research about self-serving processes (e.g., Blackwood et al., 2003; Hughes & Beer, 2012; Seidel et al., 2010), we mainly found correlations between self-attributional processes in positive and negative sentences and the precuneus, the PCC, and the insular cortex. However, our results are in line with several studies from the cognitive, social, and emotional neurosciences and with classic findings in attribution theory. Notably, we used short sentences to generate attributional processes, which is still very artificial (see the Limitations section below).
Details will be discussed in the following sections.
Task validity
On a descriptive level, our subjects more often made internal (than external) attributions for positive events during the fMRI task as well as in the IPSAQ-R, while more personal attributions were made in response to negative events. Four of the five scores in the fMRI paradigm were significantly associated with comparable results from the IPSAQ-R, including a correlation between the self-serving bias scores of both attributional tasks. Internal attributions for negative events in the fMRI paradigm and the questionnaire were unrelated. This might be due to the possibility to choose from three different attributional possibilities in the IPSAQ-R, but only from two in our task, which could have led to less pronounced attribution of negative events to internal causes in the IPSAQ-R. As is reflected in the significant correlation between the self-serving bias scores in the two tasks, the general tendencies of the subjects to attribute positive events more often to internal causes, in comparison to negative events, were similar. Thus, our results demonstrate the convergent validity of the fMRI task.
Precuneus and posterior cingulate cortex
Research in social cognitive neuroscience has demonstrated the particular functions of the precuneus and the PCC in social inferential processing (Kuzmanovic et al., 2012), such as mentalizing, intention inference, impression formation, and controlled processing (Lieberman, 2010), and the interaction between episodic memory and the processing of emotionally salient words (Maddock, 1999; Maddock, Garrett, & Buonocore, 2003) and evaluative judgments (Maddock et al., 2003; Posner et al., 2009). Furthermore, Kuzmanovic et al. reported increased neural activation of the precuneus and PCC when subjective evaluations were based on short text vignettes.
In line with the literature, our results revealed that activation in the precuneus and the PCC was associated with attributional evaluation processes of positive and negative sentences describing socially relevant situations in everyday life. Moreover, our data suggest that subregions of the precuneus and the PCC are part of different elements of these processes. The whole-brain Positive Sentences > Negative Sentences contrast revealed activations of the right PCC, whereas the reverse contrast showed activations of the left PCC. More self-attribution in both types of sentences was related to activation of the posterior portion of the precuneus, and more self-attributions of positive as compared to negative situations (the self-serving bias) revealed activation of the right anterior portion of the precuneus. Thus, activation of the PCC could be related to emotional evaluation of the presented situations, whereas activation of the posterior portion of the precuneus could be part of self-ascription processes in the context of the attributional evaluation of sentences. Furthermore, activation of the anterior portion of the precuneus might reflect functional correlates of a self-serving processes during the evaluation of positively valenced situations.
Previous studies have discussed different functions of the precuneus (for a review, see Cavanna & Trimble, 2006). One approach has assumed the participation of the precuneus in episodic memory retrieval, which was shown, for instance, by Tulving et al. (1994) and Lundstrom et al. (2003). Another idea is that the precuneus participates in self-related processes (see, e.g., Kircher et al., 2002; Kircher et al., 2000; Kjaer, Nowak, & Lou, 2002; Lou et al., 1999).
Our interpretation of a differentiation between anterior and posterior components of the precuneus refers to the review of Cavanna and Trimble (2006) and is in line with cytoarchitectonic maps (Economo & Koskinas, 1925; Scheperjans et al., 2008), as well as with previous findings from Sajonz et al. (2010). By combining tasks for self-referential processing and episodic memory, Sajonz et al. found among common networks three functional differentiations for both processes in the medial and lateral parietal cortex. One finding was the anterior–posterior differentiation in the medial parietal cortex, in particular within the precuneus and the PCC. The authors concluded that a “context-dependent neural interplay of anterior and posterior precuneus activation [is] specific for SRP [self-referential processing] and EMR [episodic memory retrieval]” (p. 1609): Activations in the antero-superior precuneus and the PCC are triggered by self-referential processes, whereas the postero-inferior activation of the precuneus is derived from EMR (Sajonz et al., 2010). Our findings support the thesis of an anterior–posterior division of the precuneus, but in a different way. On the one hand, we found evidence for the contribution of the anterior portion of the precuneus and the PCC to self-referential processes distinctly related to the valence of situations. On the other, we found a contribution to self-referential processes in the posterior portion of the precuneus, unrelated to the emotional salience of sentences.
To conclude, we suggest that the activation of the anterior portion of the precuneus is more related to self-serving tendencies, whereas the posterior portion of the precuneus is rather activated by the attribution of one’s self as being a responsible cause for social situations in general. Thus, the self-serving bias is an affective reaction, whereas general self-attribution in terms of responsibility reflects a self-referential process based on a comparison between presented situations and the memory of self-experienced events. The idea of a participation of memory processes in attribution was also put forward by Harold Kelley (e.g., 1973). Charles Antaki (1982) summarized Kelley’s concept briefly: “an attribution is arrived at by a search (but not necessarily a conscious one) for the causal candidate which is most closely associated historically with the event being explained” (p. 11). Weiner (1974) formulated as a principle for conscious causal attribution that one must “search for information, assemble and process this knowledge” (p. 56). In our task, these processes are part of the vivid imagination of our presented situations, as the portrayed events have to be compared with one’s own experiences in comparable situations. This is especially true, because our statements had no background or broader context and were presented as single sentences in an artificial experimental environment.
Insula
With increasing self-attribution of the causes of negatively, as compared to positively, valenced situations, bilateral activation in the insular cortex and the middle frontal gyrus became apparent. By using a task requiring empathy, Lamm, Batson, and Decety (2007), amongst others, found bilateral medial and anterior insula activation and left middle frontal gyrus activation when one imagines oneself in negatively valenced situations. A similar pattern can be seen in our experiment. Internally attributing negatively (as compared to positively) valenced social situations increased activation of the insula and the middle frontal gyrus. Moreover, Farrer and Frith (2002) showed the importance of the insular cortex as a contributor to one’s experiencing a sense of agency. Also, in a meta-analysis of the neural correlates of internal and external agency attributions, Sperduti et al. (2011) found bilateral insula activation as being most evident for self-agency. The central role of the insula in self-agency and self-referential processing is also supported by many studies from lesion and clinical research (e.g., Karnath & Baier, 2010; Voss et al., 2010), by meta-analyses (e.g., van der Meer et al., 2010), and by reviews (e.g., Craig, 2009; Singer et al., 2009). Furthermore, Beer and Hughes (2010) suggested that the insula is more associated with judgments of negative valence, which is in line with our finding of bilateral insular activity being correlated with self-attributed negative sentences. Although we could not control for the self-serving bias, and therefore could not prove the self-specificity of the insula activation (see Limitations, below), our findings fit with these results. Thus, we interpret our findings related to a non-self-serving bias as being correlates of assuming and accepting the responsibility for causes or being aware of oneself in negative social events.
Finally, in the context of our findings in the PCC/precuneus, our results for the insular cortex could be discussed with regard to recent findings about the default mode network (DMN). Menon and Uddin (2010), for example, suggested that the anterior insula is a core region of the salience network. According to Menon and Uddin, this network plays a key role in detecting and processing relevant environmental stimuli by triggering interactions between externally and internally oriented networks (Menon & Uddin, 2010). Furthermore, Palaniyappan and Liddle (2012) suggested that “the primary role of the salience network is initiating the recruitment of brain regions relevant for processing currently salient stimuli while decreasing activity in networks engaged in processing previously salient stimuli” (p. 23). In this context, the authors introduced the concept of proximal salience as being related to the salience network (Palaniyappan & Liddle, 2012). Here, proximal salience is understood as a temporary state of neural activity evoked by evaluating external or internal stimuli. Thereafter, proximal salience “enables a switching between resting mode to task-processing (executive) mode or vice versa” (Palaniyappan & Liddle, 2012, p. 21). Accordingly, the insula is involved in the process of updating one’s prediction model of the world by facilitating the switch between task- and non-task-related (DMN) brain areas (Palaniyappan & Liddle, 2012). In this sense, our finding of insula activity suggests that it could be more important to switch to DMN processing in situations in which internal negative attributions are assigned.
However, we did not design our study to analyze the concept of proximal salience. Furthermore, the interplay between the salience network and the DMN was not explicitly a subject of our analyses. We found higher activity in the insula for negative- than for positive-valenced situations, but we did not control for the influence of this activity on subsequent DMN activity (as this could be confounded with the bias for negative stimuli). However, our findings, along with those from future studies, might extend the notion of the salience network and its interplay with the DMN.
Limitations
Most of our limitations are due to general problems in attribution research. As Frey (1978) and Weary et al. (1982) pointed out, in public, people have a higher tendency to attribute negative outcomes to internal causes than they do in private. In line with this argument, Hewstone (1989) drew attention to the problems of public–private manipulations in the context of self-esteem and public esteem motives. Furthermore, Lloyd-Bostock (1983) pointed out that “attribution of causes and responsibility involves immensely complex processes and concepts,” but “attribution theory appears limited and narrow in emphasis” (p. 289). Thereafter, attribution of causes in a social context is partly structured by “social (including legal) rules, norms and expectations” (p. 289). Although in our task we tried to provide situations that were drawn from everyday life experiences and asked our subjects to imagine these situations vividly, our experimental conditions still had no “real-life” social environment. Moreover, by asking for an attribution and giving only two options for an answer, we experimentally reduced complex attribution possibilities that usually remain unquestioned. However, these limitations are applicable to most, if not all, emotion-related and social experiments that have been conducted in an fMRI setting or in the laboratory.
A more specific problem of our study is that, because of the variability in the design efficiency caused by unbalanced responses, we could not calculate the interaction (Internal Positive + External Negative) > (External Positive + Internal Negative). Hence, we could calculate the self-serving bias without controlling for the non-self-serving bias, and vice versa. This limits the conclusions that can be drawn from our results.
Conclusion
In line with our hypothesis, we showed that components of a fronto-temporo-parietal network are related to self-referential processes. In particular, we found that the precuneus and the PCC are related to the attributional evaluation of positive and negative sentences describing socially relevant situations in everyday life. Moreover, we could differentiate between different subregions within these brain areas. Activation of the PCC is part of emotional evaluation processes; activation of the posterior portion of the precuneus is involved in attributional evaluation processes; and activation of the anterior portion of the precuneus is related to self-serving processes evaluating the situation. In addition, we found activation of the bilateral insular cortex with increasing self-attributions of negatively, as compared to positively, valenced social situations. This may be interpreted as a correlate of the acceptance of personal agency and the awareness of oneself in negative social situations. Finally, we showed the validity of our fMRI paradigm and the comparability of its data with the IPSAQ-R.
References
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–277.
Antaki, C. (1982). A brief introduction to attribution and attributional theories. In C. Antaki & C. Brewin (Eds.), Attributions and psychological change: Applications of attributional theories to clinical and education practice (pp. 3–21). London, U.K.: Academic Press.
Beer, J. S., & Hughes, B. L. (2010). Neural systems of social comparison and the “above-average” effect. NeuroImage, 49, 2671–2679.
Blackwood, N. J., Bentall, R. P., Ffytche, D. H., Simmons, A., Murray, R. M., & Howard, R. J. (2003). Self-responsibility and the self-serving bias: An fMRI investigation of causal attributions. NeuroImage, 20, 1076–1085. doi:10.1016/S1053-8119(03)00331-8
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129, 564–583.
Cialdini, R. B., Braver, S. L., & Lewis, S. K. (1974). Attributional bias and the easily persuaded other. Journal of Personality and Social Psychology, 30, 631–637.
Craig, A. D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70.
Craig, A. D. (2010). The sentient self. Brain Structure & Function, 214, 563–577.
D’Argembeau, A., Collette, F., Van der Linden, M., Laureys, S., Del Fiore, G., Degueldre, C., & Salmon, E. (2005). Self-referential reflective activity and its relationship with rest: A PET study. NeuroImage, 25, 616–624. doi:10.1016/j.neuroimage.2004.11.048
Economo, C., & Koskinas, G. N. (1925). Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Vienna, Austria: Julius Springer.
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., & Zilles, K. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25, 1325–1335. doi:10.1016/j.neuroimage.2004.12.034
Farrer, C., Franck, N., Georgieff, N., Frith, C. D., Decety, J., & Jeannerod, M. (2003). Modulating the experience of agency: A positron emission tomography study. NeuroImage, 18, 324–333.
Farrer, C., & Frith, C. D. (2002). Experiencing oneself vs. another person as being the cause of an action: The neural correlates of the experience of agency. NeuroImage, 15, 596–603.
Fischhoff, B. (1976). Attribution theory and judgment and uncertainty. In J. H. Harvey, W. J. Ickes, & R. F. Kidd (Eds.), New directions in attribution research (Vol. 1, pp. 421–451). Hillsdale, NJ: Erlbaum.
Försterling, F. (2001). Attribution: An introduction to theories, research and applications. Hove, U.K.: Psychology Press.
Frey, D. (1978). Reactions to success and failure in public and in private conditions. Journal of Experimental Social Psychology, 14, 172–179.
Frieze, I., & Weiner, B. (1971). Cue utilization and attributional judgments for success and failure. Journal of Personality, 39, 591–605.
Garety, P. A., Hemsley, D. R., & Wessely, S. (1991). Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. Journal of Nervous and Mental Disease, 179, 194–201.
Gusnard, D. A., Akbudak, E., Shulman, G. L., & Raichle, M. E. (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences, 98, 4259–4264.
Harris, L. T., Todorov, A., & Fiske, S. T. (2005). Attributions on the brain: Neuro-imaging dispositional inferences, beyond theory of mind. NeuroImage, 28, 763–769.
Hastorf, A., Schneider, D., & Polefka, J. (1970). Person perception. Reading, MA: Addison-Wesley.
Heider, F. (1944). Social perception and phenomenal causality. Psychological Review, 51, 358–374.
Heider, F. (1958). The psychology of interpersonal relations. New York, NY: Wiley.
Hewstone, M. (1989). Causal attribution: From cognitive processes to collective beliefs. Oxford, U.K.: Blackwell.
Hughes, B. L., & Beer, J. S. (2012). Medial orbitofrontal cortex is associated with shifting decision thresholds in self-serving cognition. NeuroImage, 61, 889–898.
Iacoboni, M., Lieberman, M. D., Knowlton, B. J., Molnar-Szakacs, I., Moritz, M., Throop, C. J., & Fiske, A. P. (2004). Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage, 21, 1167–1173. doi:10.1016/j.neuroimage.2003.11.013
Johnson, S. C., Baxter, L. C., Wilder, L. S., Pipe, J. G., Heiserman, J. E., & Prigatano, G. P. (2002). Neural correlates of self-reflection. Brain, 125, 1808–1814.
Jones, E. E., & Davis, K. E. (1966). From acts to dispositions: The attribution process in person perception. In L. Berkowitz (Ed.), Advances in experimental social psychology (Vol. 2, pp. 219–266). New York, NY: Academic Press.
Jones, E. E., & Nisbett, R. E. (1987). The actor and the observer: Divergent perceptions of the causes of behavior. In E. E. Jones, D. E. Kanouse, H. H. Kelley, R. E. Nisbett, S. Valins, & B. Weiner (Eds.), Attribution: Perceiving the causes of behavior (pp. 79–94). Hillsdale, NJ: Erlbaum.
Kanouse, D. E. (1971). Language, labeling, and attribution. New York, NY: General Learning Press.
Karnath, H.-O., & Baier, B. (2010). Right insula for our sense of limb ownership and self-awareness of action. Brain Structure & Function, 214, 411–417.
Kelley, H. H. (1967). Attribution theory in social psychology. In D. Levine (Ed.), Nebraska Symposium on Motivation (Vol. 15, pp. 192–238). Lincoln, NE: University of Nebraska Press.
Kelley, H. H. (1973). The process of causal attribution. American Psychologist, 28, 107–128.
Kelley, H. H. (1987). Attribution in social interaction. Attribution: Perceiving the causes of behavior. In E. E. Jones, D. E. Kanouse, H. H. Kelley, R. E. Nisbett, S. Valins, & B. Weiner (Eds.), Attribution: Perceiving the causes of behavior (pp. 1–26). Hillsdale, NJ: Erlbaum.
Kinderman, P., & Bentall, R. P. (1996). A new measure of causal locus: The internal, personal and situational attributions questionnaire. Personality and Individual Differences, 20, 261–264.
Kircher, T. T., Brammer, M., Bullmore, E., Simmons, A., Bartels, M., & David, A. S. (2002). The neural correlates of intentional and incidental self processing. Neuropsychologia, 40, 683–692.
Kircher, T. T., Senior, C., Phillips, M. L., Benson, P. J., Bullmore, E. T., Brammer, M., & David, A. S. (2000). Towards a functional neuroanatomy of self processing: Effects of faces and words. Cognitive Brain Research, 10, 133–144.
Kjaer, T. W., Nowak, M., & Lou, H. C. (2002). Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage, 17, 1080–1086.
Klingberg, S., Wittorf, A., Meisner, C., Wölwer, W., Wiedemann, G., Herrlich, J., & Buckremer, G. (2010). Cognitive behavioural therapy versus supportive therapy for persistent positive symptoms in psychotic disorders: The POSITIVE Study, a multicenter, prospective, single-blind, randomised controlled clinical trial. Trials, 11, 123. doi:10.1186/1745-6215-11-123
Krusemark, E. A., Campbell, W. K., & Clementz, B. A. (2008). Attributions, deception, and event related potentials: An investigation of the self-serving bias. Psychophysiology, 45, 511–515. doi:10.1111/j.1469-8986.2008.00659.x
Kuzmanovic, B., Bente, G., von Cramon, D. Y., Schilbach, L., Tittgemeyer, M., & Vogeley, K. (2012). Imaging first impressions: Distinct neural processing of verbal and nonverbal social information. NeuroImage, 60, 179–188.
Lamm, C., Batson, C. D., & Decety, J. (2007). The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19, 42–58. doi:10.1162/jocn.2007.19.1.42
Lamm, C., & Singer, T. (2010). The role of anterior insular cortex in social emotions. Brain Structure & Function, 214, 579–591.
Lehrl, S. (2005). Mehrfachwahl-Wortschatz-Intelligenztest MWT-B: Fünfte unveränderte Auflage. Balingen, Germany: Spitta.
Leube, D. T., Knoblich, G., Erb, M., Grodd, W., Bartels, M., & Kircher, T. T. (2003). The neural correlates of perceiving one’s own movements. NeuroImage, 20, 2084–2090.
Lieberman, M. D. (2010). Social cognitive neuroscience. In S. T. Fiske, D. T. Gilbert, & G. Lindzey (Eds.), Handbook of social psychology (pp. 143–193). New York, NY: McGraw-Hill.
Lloyd-Bostock, S. M. (1983). Attributions of cause and responsibility as social phenomena. In J. Jaspars, F. D. Fincham, & M. Hewstone (Eds.), Attribution theory and research: Conceptual, developmental and social dimensions (pp. 261–289). New York, NY: Academic Press.
Lou, H. C., Kjaer, T. W., Friberg, L., Wildschiodtz, G., Holm, S., & Nowak, M. (1999). A 15O-H2O PET study of meditation and the resting state of normal consciousness. Human Brain Mapping, 7, 98–105.
Luginbuhl, J. E., Crowe, D. H., & Kahan, J. P. (1975). Causal attributions for success and failure. Journal of Personality and Social Psychology, 31, 86–93.
Lundstrom, B. N., Petersson, K. M., Andersson, J., Johansson, M., Fransson, P., & Ingvar, M. (2003). Isolating the retrieval of imagined pictures during episodic memory: Activation of the left precuneus and left prefrontal cortex. NeuroImage, 20, 1934–1943.
Maddock, R. J. (1999). The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences, 22, 310–316.
Maddock, R. J., Garrett, A. S., & Buonocore, M. H. (2003). Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping, 18, 30–41.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214, 655–667.
Mischel, W. (1968). Personality and assessment. New York, NY: Wiley.
Moran, J. M., Macrae, C. N., Heatherton, T. F., Wyland, C. L., & Kelley, W. M. (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience, 18, 1586–1594. doi:10.1162/jocn.2006.18.9.1586
Moritz, S., & Lincoln, T. (2008). Wahn—Psychologie. In T. Kircher & S. Gauggel (Eds.), Neuropsychologie der Schizophrenie (pp. 456–467). Heidelberg, Germany: Springer.
Moritz, S., Veckenstedt, R., Hottenrott, B., Woodward, T. S., Randjbar, S., & Lincoln, T. M. (2010). Different sides of the same coin? Intercorrelations of cognitive biases in schizophrenia. Cognitive Neuropsychiatry, 15, 406–421.
Ochsner, K. N., Knierim, K., Ludlow, D. H., Hanelin, J., Ramachandran, T., Glover, G., & Mackey, S. C. (2004). Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16, 1746–1772. doi:10.1162/0898929042947829
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. doi:10.1016/0028-3932(71)90067-4
Palaniyappan, L., & Liddle, P. F. (2012). Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry & Neuroscience, 37, 17–27.
Posner, J., Russell, J. A., Gerber, A., Gorman, D., Colibazzi, T., Yu, S., & Peterson, B. S. (2009). The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Human Brain Mapping, 30, 883–895. doi:10.1002/hbm.20553
Ridderinkhof, K. R., van den Wildenberg, W. P., Segalowitz, S. J., & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56, 129–140.
Ross, L. (1977). The intuitive psychologist and his shortcomings: Distortions in the attribution process. In L. Berkowitz (Ed.), Advances in experimental social psychology (Vol. 10, pp. 173–220). New York, NY: Academic Press.
Ruby, P., & Decety, J. (2001). Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience, 4, 546–550.
Ruby, P., & Decety, J. (2003). What you believe versus what you think they believe: A neuroimaging study of conceptual perspective-taking. European Journal of Neuroscience, 17, 2475–2480.
Sajonz, B., Kahnt, T., Margulies, D. S., Park, S. Q., Wittmann, A., Stoy, M., & Bermpohl, F. (2010). Delineating self-referential processing from episodic memory retrieval: Common and dissociable networks. NeuroImage, 50, 1606–1617. doi:10.1016/j.neuroimage.2010.01.087
Scheperjans, F., Eickhoff, S. B., Hömke, L., Mohlberg, H., Hermann, K., Amunts, K., & Zilles, K. (2008). Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cerebral Cortex, 18, 2141–2157. doi:10.1093/cercor/bhm241
Schilbach, L., Bzdok, D., Timmermans, B., Fox, P. T., Laird, A. R., Vogeley, K., & Eickhoff, S. B. (2012a). Introspective minds: Using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One, 7, e30920. doi:10.1371/journal.pone.0030920
Schilbach, L., Timmermans, B., Reddy, V., Costall, A., Bente, G., & Schlicht, T. (in press). Toward a second-person neuroscience. Behavioral and Brain Sciences.
Seidel, E. M., Eickhoff, S. B., Kellermann, T., Schneider, F., Gur, R. C., Habel, U., & Derntl, B. (2010). Who is to blame? Neural correlates of causal attribution in social situations. Social Neuroscience, 5, 335–350.
Shaver, K. G. (1975). An introduction to attribution processes. Cambridge, MA: Winthrop.
Singer, T., Critchley, H. D., & Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13, 334–340.
Sperduti, M., Delaveau, P., Fossati, P., & Nadel, J. (2011). Different brain structures related to self- and external-agency attribution: A brief review and meta-analysis. Brain Structure & Function, 216, 151–157.
Spreng, R. N., Mar, R. A., & Kim, A. S. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21, 489–510.
Stöcker, T., Schneider, F., Klein, M., Habel, U., Kellermann, T., Zilles, K., & Shah, N. J. (2005). Automated quality assurance routines for fMRI data applied to a multicenter study. Human Brain Mapping, 25, 237–246. doi:10.1002/hbm.20096
Tulving, E., Kapur, S., Markowitsch, H. J., Craik, F. I., Habib, R., & Houle, S. (1994). Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proceedings of the National Academy of Sciences, 91, 2012–2015.
van der Meer, L., Costafreda, S., Aleman, A., & David, A. S. (2010). Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews, 34, 935–946.
Vidmar, N., & Crinklaw, L. D. (1974). Attributing responsibility for an accident: A methodological and conceptual critique. Canadian Journal of Behavioural Science, 6, 112–130.
Vogeley, K., Bussfeld, P., Newen, A., Herrmann, S., Happé, F., Falkai, P., & Zilles, K. (2001). Mind reading: Neural mechanisms of theory of mind and self-perspective. NeuroImage, 14, 170–181.
Voss, M., Moore, J., Hauser, M., Gallinat, J., Heinz, A., & Haggard, P. (2010). Altered awareness of action in schizophrenia: A specific deficit in predicting action consequences. Brain, 133, 3104–3112.
Walster, E. (1966). Assignment of responsibility for an accident. Journal of Personality and Social Psychology, 3, 73–79.
Walster, E. (1967). “Second guessing” important events. Human Relations, 20, 239–249.
Weary, G., Harvey, J. H., Schwieger, P., Olson, C. T., Perloff, R., & Pritchard, S. (1982). Self-presentation and the moderation of self-serving attributional biases. Social Cognition, 1, 140–159.
Weiner, B. (1974). Achievement motivation and attribution theory. Morristown, NJ: General Learning Press.
Weiner, B. (1986). An attributional theory of motivation and emotion. New York, NY: Springer.
Wittchen, H. U., Wunderlich, U., Gruschwitz, S., & Zaudig, M. (1997). SKID-I: Strukturiertes klinisches Interview für DSM-IV. Göttingen, Germany: Hogrefe.
Zuckerman, M. (1979). Attribution of success and failure revisited or: The motivational basis is alive and well in attribution theory. Journal of Personality, 47, 245–287.
Author note
This study was funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF), Project No. 01GV0618. It is part of the BMBF research program “Research Networks on Psychotherapy.” We thank all supporting members of the fMRI subproject of the POSITIVE Study: Thilo Kellermann (RWTH Aachen); Susanne Erk, Andrea Vogeley, Julia Berning, Sarah Bluschke, Martin Landsberg, Alice Meisen, Melanie Sauder, (study site Bonn); Chistian Kärgel (study site Duisburg-Essen); Wolfgang Wölwer, Jürgen Brinkmeyer (study site Düsseldorf); Angela Ciaramidaro, Yane Eikenbusch, Swantje Merker, Kerstin Platt, Miriam Schwalm (study site Frankfurt/Main); Anna Rotarska-Jagiela, Leonhard Schilbach, Jörn Güttgemanns, Bettina Pohlmann, Tanja Tecic (study site Cologne); Andreas Wittorf, Christoph Meisner, Ruth Bösel, Ines Lengsfeld, Svenja Unsöld, (study site Tübingen); and Dominic Marjoram (University of Glasgow, U.K.). We also thank all other members of the POSITIVE Study group. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabanis, M., Pyka, M., Mehl, S. et al. The precuneus and the insula in self-attributional processes. Cogn Affect Behav Neurosci 13, 330–345 (2013). https://doi.org/10.3758/s13415-012-0143-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0143-5