Abstract
The investigation of the neural underpinnings of increased arithmetic complexity in children is essential for developing educational and therapeutic approaches and might provide novel measures to assess the effects of interventions. Although a few studies in adults and children have revealed the activation of bilateral brain regions during more complex calculations, little is known about children. We investigated 24 children undergoing one-digit and two-digit multiplication tasks while simultaneously recording functional near-infrared spectroscopy (fNIRS) and electroencephalography (EEG) data. FNIRS data indicated that one-digit multiplication was associated with brain activity in the left superior parietal lobule (SPL) and intraparietal sulcus (IPS) extending to the left motor area, and two-digit multiplication was associated with activity in bilateral SPL, IPS, middle frontal gyrus (MFG), left inferior parietal lobule (IPL), and motor areas. Oscillatory EEG data indicated theta increase and alpha decrease in parieto-occipital sites for both one-digit and two-digit multiplication. The contrast of two-digit versus one-digit multiplication yielded greater activity in right MFG and greater theta increase in frontocentral sites. Activation in frontal areas and theta band data jointly indicate additional domain-general cognitive control and working memory demands for heightened arithmetic complexity in children. The similarity in parietal activation between conditions suggests that children rely on domain-specific magnitude processing not only for two-digit but—in contrast to adults—also for one-digit multiplication problem solving. We conclude that in children, increased arithmetic complexity tested in an ecologically valid setting is associated with domain-general processes but not with alteration of domain-specific magnitude processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The investigation of the neural underpinnings of increased arithmetic complexity in children is essential for uncovering potential biomarkers to identify children at risk of mathematical learning disabilities and to develop educational and therapeutic approaches. Neuroimaging studies have shown that brain activation patterns might provide new measures to assess the effects of interventions because successful training leads to brain activation changes rather than only to behaviorally compensatory strategies (Iuculano et al., 2015). For instance, both magnitude training (Hyde, Khanum, & Spelke, 2014) and cognitive training (e.g., Witt, 2011) have been shown to improve proficiency in complex arithmetic in children. Therefore, it has been shown that neural findings are helpful for a better understanding of behavioral results (Szűcs & Goswami, 2007). However, neural correlates of problem solving at different levels of arithmetic complexity have not yet been identified in children.
Arithmetic complexity is commonly studied by investigating the contrast between multidigit and one-digit calculations. Neuroimaging studies in adults demonstrated that one-digit multiplication involves a mostly left frontoparietal network (Gruber, Indefrey, Steinmetz, & Kleinschmidt, 2001; Zago et al., 2001), whereas two-digit complex multiplication involves the intraparietal sulcus (IPS), inferior parietal lobule (IPL), angular gyrus (AG), and inferior frontal gyrus (IFG) bilaterally (Delazer et al., 2003; Delazer et al., 2005; Grabner et al., 2007; Menon, Rivera, White, Glover, & Reiss, 2000; Zago et al., 2001). Greater activation in parietal regions was interpreted as demonstrating domain-specific magnitude and quantity-based processes, that is, manipulating the numerals (e.g., Delazer et al., 2003), whereas activation in frontal regions was interpreted as signifying domain-general cognitive control and working memory processes in more complex calculations (Gruber et al., 2001; Ischebeck et al., 2006). Although there is general agreement about neural correlates of arithmetic complexity in adults, not all studies report the same findings. For instance, Rosenberg-Lee, Lovett, and Anderson (2009) suggested that arithmetic complexity, that is, more complex strategy use in this case, relies on the posterior superior parietal lobule, required for attentional demands, and on the posterior parietal cortex, for mental representation of numerals, but not on the IPS and the inferior prefrontal cortex. The findings of these adult studies, however, are not easily transferable to children due to shifts in activation from frontal to parietal areas during numerical processing tasks with increasing age and experience levels (Kaufmann, Wood, Rubinsten, & Henik, 2011; Menon, 2010; Prado, Mutreja, & Booth, 2014).
A few studies have investigated arithmetic complexity in children. In second and third graders, increased complexity of addition was associated with both domain-general cognitive processes—increased activation within the right inferior frontal sulcus and anterior insula—and domain-specific magnitude processes—increased activation within the left IPS and superior parietal lobule (SPL) regions (Rosenberg-Lee, Barth, & Menon, 2011). According to the developmental frontoparietal shift, activation of the IFG, dorsolateral, and ventrolateral prefrontal cortex decreases and activation of the left parietal cortex, supramarginal gyrus, adjoining anterior IPS, and lateral occipitotemporal cortex increases with age (e.g., Prado et al., 2014; Rivera, Reiss, Eckert, & Menon, 2005). Therefore, arithmetic complexity engages more frontal regions for younger children, who rely mostly on counting strategies, than for older children, who are more mathematically trained (see also Peters, Polspoel, de Beeck, & De Smedt, 2016; Polspoel, Peters, & De Smedt, 2016). This shows a decrease of dependency on domain-general cognitive processes with age (for a review, see Menon, 2010). Moreover, some studies have suggested a fundamental role for the hippocampal system and its connectivity to the prefrontal cortex in strategy shifts between complex and simple calculations (e.g., Cho et al., 2012), showing the pivotal role of the hippocampal system in the transition from procedural to retrieval memory-based strategies (Qin et al., 2014; Supekar et al., 2013). Altogether, studies in children suggest that more frontal engagement is associated with arithmetic complexity.
Reaching a more thorough understanding of mechanisms underlying increasing arithmetic complexity might help to develop neurobiological markers to assess responses to arithmetic trainings and interventions. For instance, Supekar et al. (2013) found that hippocampal volume and its intrinsic functional connectivity with dorsolateral and ventrolateral prefrontal cortices predicted arithmetic achievement in children, but, surprisingly, no behavioral measures were able to (for longitudinal finding, see Evans et al., 2015). To date, studies in children have usually investigated either one-digit or two-digit multiplication calculation. Further, comparisons across studies are not unequivocal, because of differences in paradigms, procedures, analysis methods, languages, and so on (Kaufmann et al., 2011; Prado et al., 2014). Therefore, we used a within-participant design in the present study to investigate the neural correlates of one-digit and two-digit multiplication problem solving in children, allowing for a direct examination of brain activity associated with increased complexity in arithmetic problem solving.
In order to address this issue in an ecologically valid setting (e.g., Obersteiner et al., 2010), a natural written production task was used in a self-paced paradigm. Two imaging techniques, functional near-infrared spectroscopy (fNIRS) and electroencephalography (EEG), were simultaneously applied in order to directly and indirectly measure neural activity underlying the processing of increased complexity in multiplication. Because of several characteristics of fNIRS, such as a reduced sensitivity to movement artifacts, which makes it particularly suitable for children and patients, this technique has been increasingly used in functional neuroimaging studies focusing on the cerebral cortex (for a review, see Ehlis, Schneider, Dresler, & Fallgatter, 2014). For EEG, the continuous data signal can be analyzed using different methods, such as event-related synchronization (ERS) and desynchronization (ERD), that is, quantificational measures of brain dynamics (Pfurtscheller & Aranibar, 1977). Studies indicate that theta and alpha frequency bands behave in opposite ways in response to cognitive tasks such as arithmetic processing (e.g., Dolce & Waldeier, 1974). For instance, task complexity, attentional and cognitive demands, and memory load lead to theta ERS (increase in theta power) but also cause alpha ERD (decrease in alpha power; Antonenko, Paas, Grabner, & van Gog, 2010; Gevins, Smith, McEvoy, & Yu, 1997; Klimesch, 1999; Pfurtscheller, Stancak, & Neuper, 1996; Pfurtscheller & Da Silva, 1999). Furthermore, some cognitive functions are more closely linked to one of these frequency bands. In particular, it has been reported that the theta band reflects the encoding of new information, whereas the alpha band reflects searching for and retrieving information from long-term semantic memory storage (Antonenko et al., 2010; Jensen & Tesche, 2002; Klimesch, 1999; Sammer et al., 2007; Sauseng & Klimesch, 2008). In numerical cognition, some studies interpreted the theta frequency band as a sign of domain-general cognitive demands of arithmetic processing, such as sustained attention and working memory, and the alpha frequency band as an indicator of fact retrieval from long-term memory in different arithmetic tasks (Harmony et al., 1999; Klados et al., 2013; Micheloyannis, Sakkalis, Vourkas, Stam, & Simos, 2005; Mizuhara & Yamaguchi, 2007; Moeller, Wood, Doppelmayr, & Nuerk, 2010). However, other studies interpreted the theta band as a function of arithmetic fact retrieval processes and the alpha band as a function of procedural processes (De Smedt, Grabner, & Studer, 2009; Grabner & De Smedt, 2011, 2012). Therefore, using fNIRS simultaneously with EEG may help to more consistently interpret the findings of brain dynamic changes recorded by oscillatory EEG signals in arithmetic processing (see also Sammer et al., 2007).
Given previous findings, we hypothesize greater domain-specific magnitude processes for two-digit than for one-digit multiplication, which should lead to extensive activation in parietal regions in fNIRS data, potentially more left lateralized (Chochon, Cohen, Van De Moortele, & Dehaene, 1999; Kazui, Kitagaki, & Mori, 2000; Rickard et al., 2000). Moreover, additional domain-general cognitive demands for two-digit compared to one-digit multiplication are expected, which should result in activation in frontal regions in fNIRS data and greater theta ERS in EEG data (see also Micheloyannis et al., 2005). Note that in order to measure arithmetic complexity in an ecologically valid situation, the written production paradigm was used in the present study, which might lead to greater motor responses and irrelevant brain activation changes in the motoric areas compared to more common paradigms such as verification. Additionally, because of considerable interindividual differences in children (Siegler, 1988; for a review, see De Smedt, 2015), and the contribution of domain-general cognitive factors to these differences (Nemati et al., 2017; Vanbinst, Ghesquiere, & De Smedt, 2014), the role of memory components (for a review, see Menon, 2016) and strategy use (e.g., Grabner & De Smedt, 2011) in multiplication performance was assessed (for a review, see Vanbinst & De Smedt, 2016).
Material and methods
Participants
Twenty-six typically developing fifth-grade children participated in the study. No child had a history of neurological or mental disorders. Due to technical problems during EEG recording, two children were excluded: for one child, the connection failed between the recorder and computer presenting the task, and in the other child, no trigger was recorded by the EEG recorder. The remaining 24 children (nine girls; age 11.1 ± 0.5 years) were right-handed with normal or corrected-to-normal vision. Informed consent was obtained from all children and parents included in the study. They received expense allowance for participation. The study was approved by the Ethics Commission of the University Hospital of Tuebingen.
Material
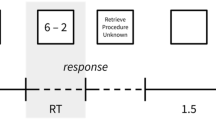
Sixteen one-digit and 16 two-digit multiplication problems were used. The one-digit problems (e.g., 3 × 9) included two one-digit operands (range 2–9) with two-digit solutions (range 12–40). The two-digit problems (e.g., 18 × 4) included two-digit (range 12–19) times one-digit operands (range 3–8) with two-digit solutions (range 52–98). The order of small and large operands was counterbalanced in both conditions. Problems with ones (e.g., 8 × 1), commutative pairs (e.g., 18 × 4 and 4 × 18), or ties (4 × 4) were not used. The experiment was run using Presentation® software (Version 16.3, Neurobehavioral Systems Inc., www.neurobs.com). Multiplication problems were presented on the screen in white font against a black background (see Fig. 1a). Responses were recorded via written production, which children typically use to perform arithmetic tasks in school.
a Multiplication problems: In a production paradigm, the problems were presented on the left side of the screen until the participant pressed the gray box or the maximal response time was reached. b Schematic positions of fNIRS optodes and EEG electrodes: The red circles indicate emitters and blue circles indicate detectors in the two arrays of 3 × 5. Small white shapes indicate positions of the EEG electrodes. Red dotted shapes indicate the original position of some EEG electrodes according to the international 10/20 system. c Experimental setting: Children wrote their responses on the touch screen (Color figure online)
FNIRS
FNIRS data were collected with the ETG 4000 Optical Topography System (Hitachi Medical Co., Tokyo, Japan), which uses two wavelengths (695 and 830 nm) to calculate the absorption changes in oxygenated (O2Hb) and deoxygenated hemoglobin (HHb) concentration using a modified Beer-Lambert law. The sampling rate was 10 Hz, and the interoptode distance was 30 mm. Fifteen optodes (eight emitters, seven detectors) in a 3 × 5 arrangement were attached to an elastic combined fNIRS-EEG cap (Brain Products GbmH., Herrsching, Germany) over both hemispheres, resulting in 22 measurement channels per hemisphere (cf. Fig. 1b). Channel 14 (left hemisphere) was placed over the P3 electrode site, and channel 18 (right hemisphere) was placed over P4 in accordance with the international 10/20 system (Jasper, 1958). The localization of the corresponding cortical areas (Singh, Okamoto, Dan, Jurcak, & Dan, 2005; Tsuzuki et al., 2007) is based on the AAL (automatic anatomical labeling) atlas (Tzourio-Mazoyer et al., 2002) in SPM software (http://www.fil.ion.ucl.ac.uk/spm).
EEG
EEG was recorded from 21 scalp electrodes also embedded into the combined fNIRS-EEG cap (cf. Fig. 1b). Given the fixed optode distances, EEG electrodes were placed according to the extended international 10/20 system (Jasper, 1958; Oostenveld & Praamstra, 2001). To identify eye movement artifacts in the EEG signal, electrooculography (EOG) was recorded from an additional electrode below the right eye. The ground electrode was placed on AFz and the online reference electrode on FCz. Electrode impedance was kept below 20 kΩ. EEG data were recorded using a 32-channel DC amplifier and the Brain Vision Recorder software (Brain Products GmbH., Herrsching, Germany). Data were digitized at a rate of 1000 Hz with an online band-pass filter of 0.1–100.0 Hz.
Neuropsychological tests
Intelligence was measured using the similarities and matrix reasoning subtests of the German Wechsler intelligence quotient (IQ) test (Petermann, Petermann, & Wechsler, 2007). Due to time constraints, we only used these two subtests to control for general verbal and performance intelligence of the participants. Four memory components were assessed (Alloway, Gathercole, & Pickering, 2006). The letter span test was used to measure verbal short-term memory, and the block tapping task (Corsi, 1973) was used to assess visuospatial short-term memory. For these verbal and visuospatial working memory tasks, children were required to recall sequences of letters or cubes inversely. In general, forward span tests were defined as short-term memory and backward span tests were defined as working memory (Cowan, 2008; for more, see Soltanlou, Pixner, & Nuerk, 2015). To assess strategies used in solving one-digit and two-digit problems, we designed a strategy questionnaire, which was completed by children before the experiment. The questionnaire contained four one-digit and four two-digit experimental problems, resulting in four matched versions with different problems each. After responding to each problem, children reported how they solved it. The reported strategies were categorized as retrieval, procedural, and other for the analysis (see also Grabner & De Smedt, 2011). The interrater reliability, which was calculated by Cohen’s kappa, was 0.80.
Procedure
All children were tested individually while seated comfortably in front of the touch screen in a light-attenuated room. During the 45-minute preparation of the combined fNIRS-EEG cap (cf. Fig. 1c) by two experimenters, children watched a cartoon. Before the actual experiment, the children completed four practice trials. Children were tested on a computerized written production paradigm in which problems were presented without response options and children had to produce the solution as quickly and accurately as possible. They were instructed to read the problems silently and calculate mentally. As soon as they found the solution, they wrote it down on the touch screen with the help of a touch pen and then clicked on a gray box to continue (see Fig. 1a). Note that the written response was not visible on the screen, to avoid any further correction. The task was self-paced with a limited response interval of 10 s for one-digit problems and 30 s for two-digit problems. Therefore, due to interindividual differences, the number of solved problems differed between children. The intertrial interval was set to 0.5 s. The experiment was a block design, and the multiplication problems of each condition were presented in 16 blocks (eight for one-digit and eight for two-digit multiplication) of 45 s followed by 20 s of rest, resulting in a total experiment duration of approximately 18 minutes. The sequence of the blocks and of the problems was randomized. Whenever the total number of trials within a condition was reached, the same problems were presented again after randomization. No feedback was given.
Because this study was part of a larger training project that required two visits to the laboratory, the strategy questionnaire was completed during the first visit, and the IQ and memory tests were conducted during the second visit.
Analysis
Behavioral data
Response times (RTs) were defined as the time from stimulus onset to pressing the gray box after a written response. Only RTs for correct responses were entered into the analyses. Error rate was defined as proportion of incorrect and missed trials to total number of presented trials. Written responses by participants were read with the help of a RON (ReadOutNumbers) program (Ploner, 2014) to calculate error rates. Mean RTs and arcsine-square-root-transformed error rate, applied to approximate normal distribution (Winer, Brown, & Michels, 1971), between two conditions were compared using paired t tests. Relation of behavioral data with neuropsychological data was analyzed using bivariate correlation. The analysis was completed using SPSS Version 23.0 (IBM Corp.).
FNIRS
The continuous concentration changes of O2Hb and HHb were recorded for 22 channels per hemisphere. Hemoglobin quantity was scaled in mM*mm, which is based on the idea that concentration changes depend on the path length of NIR light through the brain. Data were analyzed using a commercial software package, MATLAB (MathWorks, Natick, MA). Signals were band-pass filtered with 0.008–0.25 Hz, and large motion artifacts and nonevoked systemic influences, such as heart rate and very low frequency oscillations, were reduced using the correlation-based signal improvement (CBSI) method (Cui, Bray, & Reiss, 2010). Afterwards, this CBSI time course, which is based on an expected negative correlation of concentration changes of O2Hb and HHb, was used to indicate cortical activation. For every participant, remaining noisy channels were interpolated using the mean of the surrounding channels. The amplitude of each 45 s block was baseline-corrected using the 2 s before the respective block and averaged for each condition and participant. To investigate the brain activation in each condition, t tests against zero were calculated, and a paired t test was applied to assess the contrast of two-digit versus one-digit multiplication. The significance level was .05 and corrected using the Dubey/Armitage-Parmar (D/AP) method for multiple comparisons (Sankoh, Huque, & Dubey, 1997). D/AP is among the stepwise modified Bonferroni procedures that consist of readjusting the level of significance for the individual test while taking into account autocorrelations in the data. This procedure is well suited to the analysis of fNIRS data, due to the usually strong correlations between neighboring fNIRS channels. Furthermore, to look at the lateralization of activation, the average amplitudes of the left and right hemispheres were compared for each condition using paired t tests.
EEG
EEG data were analyzed using the Brainstorm toolbox (Tadel, Baillet, Mosher, Pantazis, & Leahy, 2011), a documented and freely available software (http://neuroimage.usc.edu/brainstorm). Data were offline rereferenced to average reference and filtered using a band pass of 0.1–40.0 Hz. Then, eye movement artifacts were detected based on the EOG signal with the peak beyond 2 standard deviations of the mean, and were removed using Signal Space Projections (SSP) from the continuous signal of EEG electrodes. Epochs of 45 s experimental and 20 s rest intervals were used for analysis. For frequency analysis, the power spectral density (PSD) for theta (4.1–7.0 Hz) and alpha (7.1–13.0 Hz), two frequently investigated frequency bands in cognitive tasks (Antonenko et al., 2010), was calculated. The PSDs of every epoch were calculated separately and averaged for each condition and participant, resulting in three PSDs per participant (for one-digit multiplication, two-digit multiplication, and rest). In the next step, ERS/ERD were calculated, which are related to cortical activation and functional changes of brain activity (Neuper & Klimesch, 2006; Pfurtscheller & Da Silva, 1999). Because of several factors that influence EEG variation, such as individual differences, age (Klimesch, 1999), and differences in brain volume (Nunez & Cutillo, 1995), it is recommended that investigators analyze changes in the EEG, rather than analyzing the absolute power of each frequency band, in order to increase the reliability of findings (Pfurtscheller & Da Silva, 1999). According to the expression ERS/ERD% = (PSD of activation – PSD of rest)/PSD of rest × 100 (Pfurtscheller & Da Silva, 1999), the percentage value for ERS/ERD for each of the multiplication conditions was calculated for every participant. If the PSD of a condition is larger than rest, the result will be positive, indicating ERS, while negative differences indicate ERD. For each condition, statistical analyses consisted of t tests against zero for ERS/ERD% for each electrode and each frequency band. To investigate the contrast of conditions, paired t tests were applied with a significance level of .001 and corrected for multiple comparisons using the Bonferroni method. Note that EEG electrodes record the average oscillations of the whole brain—including almost all cortical and subcortical structures—at each recording site, whereas fNIRS records the average reflected light from a maximum of approximately 3 cm surrounding cortical and subcortical structures. Therefore, to control the Type I error, we used a more conservative correction method (i.e., Bonferroni) on EEG data than on fNIRS data.
Results
Behavioral data
Children were faster at solving one-digit (4.77 s, SD = 0.89 s) than two-digit multiplication problems (10.73 s, SD = 2.61 s), t(23) = 13.79, p < .001. They also made fewer errors in one-digit (15.34%, SD = 7.06%) than in two-digit problems (29.08%, SD = 11.37%), t(23) = 8.09, p < .001.
FNIRS
In one-digit multiplication, left SPL, IPS, and postcentral gyrus displayed significant activation, t(23) > 3.09, corrected p < .05, which extended to the precentral motor cortex. Moreover, significant deactivation was observed in left superior temporal gyrus, right superior and middle temporal gyri, precentral gyrus and IFG, t(23) < -2.64, corrected p < .05 (cf. Fig. 2). In two-digit multiplication, bilateral SPL, IPS, and MFG, along with left IPL, postcentral and precentral gyri displayed significant activation, t(23) > 2.84, corrected p < .05. Moreover, significant deactivation was observed in the right superior and middle temporal gyri, and precentral gyrus, t(23) < -3.22, corrected p < .05 (see Fig. 2).
The contrast between two-digit and one-digit multiplication revealed significantly stronger activation for the right MFG, t(23) > 3.02, corrected p < .05, extending into the IFG (cf. Fig. 2). Additionally, a significantly greater activation was found in the left compared to the right hemisphere in both one-digit, t(23) = 3.19, p < .01, and two-digit multiplication, t(23) = 3.79, p < .01.
EEG
In both one-digit and two-digit multiplication, theta ERS and alpha ERD were observed during the experiment. Theta band activity was found to be significantly above zero in middle and right occipitoparietal sites (Oz and O2 electrodes) in one-digit multiplication, t(23) > 6.41, corrected p < .001. The same significant activity was observed in bilateral occipitoparietal sites extending to the right temporal site (O1, O2, and TPP8h electrodes) in two-digit multiplication, t(23) > 5.55, corrected p < .001. These results suggest stronger theta power in these sites during the experiment than in rest intervals. Regarding alpha ERD, a significant difference from zero in the alpha band was found in bilateral occipitoparietal sites (O1 and O2 electrodes) for both conditions, t(23) < -6.32, corrected p < .001. These results suggest lower alpha power in these sites during the experiment than in rest intervals (see Fig. 3a).
a Theta ERS and alpha ERD in one-digit and two-digit multiplication problems (red means ERS and blue means ERD). b The difference of theta ERS and alpha ERD over all electrodes in one-digit and two-digit multiplication. A significant increase in theta ERS was found for two-digit versus one-digit multiplication (marked by *) (Color figure online)
Based on prior studies (e.g., Ishii et al., 2014) that have found frontal midline theta increase during focused attention on mental calculation, we examined whether there would be a significant difference in theta ERS between two-digit and one-digit multiplication. We observed greater theta ERS in frontocentral sites (Fz and Cz electrodes) during two-digit than in one-digit multiplication, t(23) > 2.12, p < .05, but it did not survive correction for multiple comparisons. No significant difference was found between the two conditions in the alpha band. Furthermore, in the contrast of two-digit versus one-digit multiplication over all electrodes, a significant difference was observed in theta ERS, t(23) = 1.98, p = .03, but not in alpha ERD (see Fig. 3b).
Reanalysis of fNIRS and EEG data by adding RTs and error rates as covariates
As regards fNIRS, ANCOVA analysis showed no significant activation or deactivation in the one-digit or two-digit condition after correction for multiple comparisons. The contrast of two conditions was not significant.
With respect to EEG, ANCOVA analysis displayed no significant difference in alpha and theta bands in the one-digit or two-digit condition. The contrast of two conditions was not significant.
Neuropsychological tests
The performance of children in the similarities and matrix reasoning subtests of the IQ test was within a normal range (cf. Table 1). Additional information regarding memory tests and strategy use are displayed in Table 1. Children reported significantly more retrieval strategy use, t(23) = 4.66, p < .001, and less procedural strategy use, t(23) = -3.99, p < .001, to solve one-digit versus two-digit multiplication.
Because of interindividual differences among children, correlation analyses between behavioral and neuropsychological data were conducted to investigate whether these neuropsychological factors influenced multiplication performance. We found that children with better visuospatial short-term and working memory were faster and made fewer errors in one-digit multiplication (see Table 2). Furthermore, children who reported higher reliance on a retrieval strategy in one-digit multiplication were faster in solving these problems, and children who reported higher reliance on procedural strategies were slower in responding to one-digit multiplication problems (cf. Table 2).
Discussion
In the present study, the neural underpinnings of increased multiplication complexity were investigated with simultaneous fNIRS-EEG in children in a within-participant design. Behavioral findings revealed faster and more accurate responses in solving one-digit than in solving two-digit multiplication problems, which is congruent with the greater use of retrieval and fast compact procedural strategies for these problems (Lemaire & Siegler, 1995). Following previous findings showing that children use various strategies for solving one-digit multiplication (Cooney, Swanson, & Ladd, 1988; Lemaire & Siegler, 1995), children used both retrieval and procedural strategies. Further, domain-general capabilities (i.e., visuospatial short-term and working memory) contribute to one-digit multiplication performance (see also Ashkenazi, Rosenberg-Lee, Metcalfe, Swigart, & Menon, 2013; Soltanlou et al., 2015).
During one-digit multiplication, activation was observed in the left SPL and IPS, while theta ERS and alpha ERD were observed over occipitoparietal regions. These activation patterns have already been reported in multiplication problem solving in adults (Chochon et al., 1999; Dehaene et al., 1996; Delazer et al., 2003; Kawashima et al., 2004; Kazui et al., 2000; Micheloyannis et al., 2005; Rickard et al., 2000; Zago et al., 2001; Zhou et al., 2007). Both theta ERS and alpha ERD in solving one-digit multiplication are also in line with neurophysiological changes in multiplication problem solving in adults (Micheloyannis et al., 2005). The findings suggest that children in this developing age still rely on quantity-based knowledge, aside from arithmetic fact retrieval, to solve one-digit multiplication problems (but see Kawashima et al., 2004), a conclusion that is additionally supported by the reported strategy use (see also Lemaire & Siegler, 1995). It has been shown that even adults do not always retrieve solutions but rather use several back-up strategies (e.g., for large one-digit multiplication problems; LeFevre et al., 1996; Zhou et al., 2007).
Contrary to studies in adults (e.g., Delazer et al., 2003; Grabner et al., 2007) and a few studies in children (Cho et al., 2012; Peters et al., 2016), the activation of the left AG, which has been associated with retrieval strategies, was not observed in the present study. Delazer et al. (2005) found that depending on the strategy use, retrieval processes were associated with bilateral occipitoparietal areas including the precuneus (see also Andres, Pelgrims, Michaux, Olivier, & Pesenti, 2011; Prado et al., 2013) and not necessarily with the left AG. Note that previous studies in children found AG activation in small one-digit addition and subtraction problems (Cho et al., 2012; Peters et al., 2016), whereas in the present study the whole range of one-digit multiplication was used, which probably led to an overall increase in procedural processes in the one-digit condition (for more discussion about the AG, see Grabner, Ansari, Koschutnig, Reishofer, & Ebner, 2013).
In two-digit multiplication, bilateral activation of the SPL, IPS, MFG, and left IPL were observed as well as posterior theta ERS extending to right temporal sites and alpha ERD over occipitoparietal sites (see also Grabner & De Smedt, 2011, 2012). Complex multiplication problems are usually solved via procedural step-by-step calculations (for a review, see Zamarian, Ischebeck, & Delazer, 2009), which recruit the bilateral frontoparietal network (Gruber et al., 2001; Delazer et al., 2003; Delazer et al., 2005; Ischebeck et al., 2006). These procedural processes might be related to the observed theta ERS, which was stronger during two-digit than one-digit multiplication problem solving. This was in line with the findings by Micheloyannis et al. (2005), who reported stronger theta ERS during complex multiplication problem solving in adults.
In regard to increased multiplication complexity, the children showed larger activation of right MFG and IFG (see also Fehr, Code, & Herrmann, 2007). This might be interpreted as reflecting the additional involvement of domain-general cognitive demands, such as working memory, sustained attention, and planning, in two-digit as opposed to one-digit calculation (Fehr et al., 2007; Gruber et al., 2001; Zago et al., 2001), since activation of the frontal cortex has been shown to be related to cognitive control and working memory (Cabeza & Nyberg, 2000; Ranganath, Johnson, & D’Esposito, 2003; Sylvester et al., 2003). Rivera et al. (2005) showed that older children, who solve arithmetic problems faster and more accurately than younger children, rely less on frontal regions (see also Prado et al., 2014). This finding is partially in line with the study by Rosenberg-Lee et al. (2011), which found frontal activation to be related to greater cognitive load in more complex calculations. However, in contrast to their findings, no significant activation of parietal cortex was observed related to the increased complexity. In the present study, the most commonly reported procedural strategy used in two-digit multiplication was separately multiplying the unit and decade digits of the two-digit operand with the one-digit operand (relying on retrieval strategies) and adding the results together (see also Tschentscher & Hauk, 2014). In this procedure, each step needs to be kept in working memory, and different cognitive control elements are involved, such as inhibiting operand-related mistakes, and performing self-monitoring and error detection during each step of calculation. Note that although some studies in children suggest a transitional role of the hippocampus in arithmetic development (e.g., Cho et al., 2012; Qin et al., 2014; Supekar et al., 2013), fNIRS is not capable of recording activation within subcortical and other nonsurface structures.
Greater theta ERS with increased multiplication complexity is in line with a similar study in adults (Micheloyannis et al., 2005). Theta oscillations among frontal areas have been reported to originate from a cortico-hippocampal network and the medial prefrontal area (Klimesch, 1996, 1999; Mizuhara & Yamaguchi, 2007; Sauseng & Klimesch, 2008). Furthermore, simultaneous fMRI-EEG studies of subtraction (Mizuhara & Yamaguchi, 2007) and addition (Sammer et al., 2007) reported theta ERS over frontal areas as a function of cognitive control, working memory, encoding, and self-monitoring. Nonetheless, increased multiplication complexity did not lead to a difference in alpha ERD as reported for adults (Micheloyannis et al., 2005). Alpha ERD has been suggested to be related to several cognitive functions including retrieving information from long-term memory (Antonenko et al., 2010; Harmony et al., 1999; Klimesch, 1999; Moeller et al., 2010). Therefore, we conclude that this similar pattern of alpha ERD is related to retrieval strategy use not only in one-digit multiplication but also as part of an algorithm procedure in two-digit multiplication. By replicating the findings of Micheloyannis et al. (2005) and extending them to children, we conclude that theta ERS is more related to procedural strategies and additional cognitive processes, and alpha ERD is mostly related to retrieval processes in mental calculation (see also Harmony et al., 1999; Jensen & Tesche, 2002; Kahana, Seelig, & Madsen, 2001; Klimesch, 1999; Mizuhara & Yamaguchi, 2007; but see De Smedt et al., 2009; Grabner & De Smedt, 2011, 2012). Note that because the difference between one-digit and two-digit calculations is not very large, the contrast of two conditions does not survive correction for multiple comparisons and must be interpreted cautiously. However, this contrast interestingly corroborates previous ERD/ERS studies of arithmetic processing.
The activation of the left motor cortex may be explained by the type of response production because all children responded with their right hand. Furthermore, it should be noted that although children were asked to calculate silently, it is possible that they were doing (additional) step-by-step calculation via inner speech, which may lead to the same results as subvocalization of the answers in silent verbal production tasks (e.g., Dehaene et al., 1996), and a trace of finger counting could have been present during mental calculation (Delazer et al., 2003; Zago et al., 2001). In regard to the lateralization of brain activation, in line with previous studies of multiplication in adults (Chochon et al., 1999; Dehaene et al., 1996; Kazui et al., 2000; Rickard et al., 2000; Zago et al., 2001), we found stronger activation in the left hemisphere for both one-digit and two-digit multiplication, which is assumed to reflect language-related processes in solving multiplication (Dehaene, Molko, Cohen, & Wilson, 2004). However, it should be mentioned that direct comparisons of fNIRS data stemming from different brain hemispheres is difficult due to the different path lengths the light travels depending on anatomical characteristics of the underlying brain areas (see Katagiri et al., 2010; Zhao et al., 2002), which might explain a part of this difference.
We conducted additional ANCOVA by adding response times and error rates to the model. In the present block-designed study with a self-paced written production paradigm, the covariates, particularly response times, seemed to subserve the cognitive processes underlying the performance. Therefore, using response times as a covariate may not methodologically represent the best approach (see Miller & Chapman, 2001). As conditions and response times are highly correlated, this may be a major problem for ANCOVA application. One often untested prerequisite of ANCOVAs is that the independent variable (i.e., condition) and covariate (i.e., response times) do not share a common variance. If they do, then it should be ensured that dependence arises just by chance (e.g., due to randomization processes). If dependence results not from chance, but from an inherent dependence of the two variables, then an ANCOVA may conceal effects that are actually there, or may even introduce nonexisting effects (for a more thorough discussion, see Miller & Chapman, 2001). As complexity influences both activation and response time, partialling out either dependent variable may result in biased effects. In sum, because the response time prolongation and the activation are subserved by the same neurocognitive processes, a closer look at significant regions in a t test that are nonsignificant in an ANCOVA might be instructive.
Conclusions
Both activation patterns in frontal cortex and theta band data indicate that in children, increased multiplication complexity requires domain-general processing, or additional demands on cognitive control and working memory, consistent with the literature.
However, contrary to previous results in and conclusions reached from adults, the lack of a difference in activation patterns in SPL and IPS suggests that children in this developing age still rely on magnitude processing for both one-digit and two-digit multiplication problem solving. This finding is new since increased multiplication complexity in children tested in an ecologically valid setting is associated with additional cognitive load, but not with additional magnitude processing, as in previous adult studies. Interventions based on adult neuroimaging results may therefore be suboptimal. We suggest that to improve interventional and educational approaches for arithmetic complexity during development, neurocognitive studies with children are needed, ideally with simultaneous recording with fNIRS and EEG to reach integrated conclusions for development and intervention.
References
Alloway, T. P., Gathercole, S. E., & Pickering, S. J. (2006). Verbal and visuospatial short‐term and working memory in children: Are they separable? Child development, 77(6), 1698–1716.
Andres, M., Pelgrims, B., Michaux, N., Olivier, E., & Pesenti, M. (2011). Role of distinct parietal areas in arithmetic: an fMRI-guided TMS study. NeuroImage, 54(4), 3048–3056.
Antonenko, P., Paas, F., Grabner, R., & van Gog, T. (2010). Using electroencephalography to measure cognitive load. Educational Psychology Review, 22(4), 425–438.
Ashkenazi, S., Rosenberg-Lee, M., Metcalfe, A. W., Swigart, A. G., & Menon, V. (2013). Visuo–spatial working memory is an important source of domain-general vulnerability in the development of arithmetic cognition. Neuropsychologia, 51(11), 2305–2317.
Cabeza, R., & Nyberg, L. (2000). Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience, 12(1), 1–47.
Cho, S., Metcalfe, A. W., Young, C. B., Ryali, S., Geary, D. C., & Menon, V. (2012). Hippocampal–prefrontal engagement and dynamic causal interactions in the maturation of children’s fact retrieval. Journal of cognitive neuroscience, 24(9), 1849–1866.
Chochon, F., Cohen, L., Van De Moortele, P., & Dehaene, S. (1999). Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience, 11(6), 617–630.
Cooney, J. B., Swanson, H. L., & Ladd, S. F. (1988). Acquisition of mental multiplication skill: Evidence for the transition between counting and retrieval strategies. Cognition andIinstruction, 5(4), 323–345.
Corsi, P. M. (1973). Human memory and the medial temporal region of the brain. Ann Arbor, MI: ProQuest Information & Learning.
Cowan, N. (2008). What are the differences between long-term, short-term, and working memory? Progress in Brain Research, 169, 323–338.
Cui, X., Bray, S., & Reiss, A. L. (2010). Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage, 49(4), 3039–3046.
De Smedt, B. (2015). Individual differences in arithmetic fact retrieval. In D. Berch, D. Geary, & K. Mann Koepke (Eds.), Development of mathematical cognition: Neural substrates and genetic influences (Vol. 2, pp. 219–243). Cambridge, MA: Academic Press.
De Smedt, B., Grabner, R. H., & Studer, B. (2009). Oscillatory EEG correlates of arithmetic strategy use in addition and subtraction. Experimental Brain Research, 195(4), 635–642.
Dehaene, S., Molko, N., Cohen, L., & Wilson, A. J. (2004). Arithmetic and the brain. Current Opinion in Neurobiology, 14(2), 218–224.
Dehaene, S., Tzourio, N., Frak, V., Raynaud, L., Cohen, L., Mehler, J., & Mazoyer, B. (1996). Cerebral activations during number multiplication and comparison: A PET study. Neuropsychologia, 34(11), 1097–1106.
Delazer, M., Domahs, F., Bartha, L., Brenneis, C., Lochy, A., Trieb, T., & Benke, T. (2003). Learning complex arithmetic—An fMRI study. Cognitive Brain Research, 18(1), 76–88.
Delazer, M., Ischebeck, A., Domahs, F., Zamarian, L., Koppelstaetter, F., Siedentopf, C., … & Felber, S. (2005). Learning by strategies and learning by drill—Evidence from an fMRI study. NeuroImage, 25(3), 838–849.
Dolce, G., & Waldeier, H. (1974). Spectral and multivariate analysis of EEG changes during mental activity in man. Electroencephalography and Clinical Neurophysiology, 36, 577–584.
Ehlis, A.-C., Schneider, S., Dresler, T., & Fallgatter, A. J. (2014). Application of functional near-infrared spectroscopy in psychiatry. NeuroImage, 85, 478–488.
Evans, T. M., Kochalka, J., Ngoon, T. J., Wu, S. S., Qin, S., Battista, C., & Menon, V. (2015). Brain structural integrity and intrinsic functional connectivity forecast 6 year longitudinal growth in children’s numerical abilities. The Journal of Neuroscience, 35(33), 11743–11750.
Fehr, T., Code, C., & Herrmann, M. (2007). Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI–BOLD activation. Brain Research, 1172, 93–102.
Gevins, A., Smith, M. E., McEvoy, L., & Yu, D. (1997). High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex, 7(4), 374–385.
Grabner, R. H., Ansari, D., Koschutnig, K., Reishofer, G., & Ebner, F. (2013). The function of the left angular gyrus in mental arithmetic: Evidence from the associative confusion effect. Human Brain Mapping, 34(5), 1013–1024.
Grabner, R. H., Ansari, D., Reishofer, G., Stern, E., Ebner, F., & Neuper, C. (2007). Individual differences in mathematical competence predict parietal brain activation during mental calculation. NeuroImage, 38(2), 346–356.
Grabner, R. H., & De Smedt, B. (2011). Neurophysiological evidence for the validity of verbal strategy reports in mental arithmetic. Biological Psychology, 87(1), 128–136.
Grabner, R. H., & De Smedt, B. (2012). Oscillatory EEG correlates of arithmetic strategies: A training study. Frontiers in Psychology, 3. doi:10.3389/fpsyg.2012.00428
Gruber, O., Indefrey, P., Steinmetz, H., & Kleinschmidt, A. (2001). Dissociating neural correlates of cognitive components in mental calculation. Cerebral Cortex, 11(4), 350–359.
Harmony, T. A., Fernández, T. A., Silva, J., Bosch, J., Valdés, P., Fernández-Bouzas, A., … & Rodrı́guez, D. (1999). Do specific EEG frequencies indicate different processes during mental calculation? Neuroscience Letters, 266(1), 25–28.
Hyde, D. C., Khanum, S., & Spelke, E. S. (2014). Brief non-symbolic, approximate number practice enhances subsequent exact symbolic arithmetic in children. Cognition, 131(1), 92–107.
Ischebeck, A., Zamarian, L., Siedentopf, C., Koppelstätter, F., Benke, T., Felber, S., & Delazer, M. (2006). How specifically do we learn? Imaging the learning of multiplication and subtraction. NeuroImage, 30(4), 1365–1375.
Ishii, R., Canuet, L., Ishihara, T., Aoki, Y., Ikeda, S., Hata, M., … & Nakahachi, T. (2014). Frontal midline theta rhythm and gamma power changes during focused attention on mental calculation: An MEG beamformer analysis. Frontiers in Human Neuroscience, 8, 406. doi:10.3389/fnhum.2014.00406
Iuculano, T., Rosenberg-Lee, M., Richardson, J., Tenison, C., Fuchs, L., Supekar, K., & Menon, V. (2015). Cognitive tutoring induces widespread neuroplasticity and remediates brain function in children with mathematical learning disabilities. Nature Communications, 6. Retrieved from https://www.nature.com/articles/ncomms9453
Jasper, H. H. (1958). The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10, 371–375.
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399.
Kahana, M. J., Seelig, D., & Madsen, J. R. (2001). Theta returns. Current Opinion in Neurobiology, 11(6), 739–744.
Katagiri, A., Dan, I., Tuzuki, D., Okamoto, M., Yokose, N., Igarashi, K., … & Yamaguchi, Y. (2010). Mapping of optical pathlength of human adult head at multi-wavelengths in near infrared spectroscopy Oxygen Transport to Tissue XXXI (pp. 205–212). New York, NY: Springer.
Kaufmann, L., Wood, G., Rubinsten, O., & Henik, A. (2011). Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Developmental Neuropsychology, 36(6), 763–787.
Kawashima, R., Taira, M., Okita, K., Inoue, K., Tajima, N., Yoshida, H., … & Fukuda, H. (2004). A functional MRI study of simple arithmetic—A comparison between children and adults. Cognitive Brain Research, 18(3), 227–233.
Kazui, H., Kitagaki, H., & Mori, E. (2000). Cortical activation during retrieval of arithmetical facts and actual calculation: A functional magnetic resonance imaging study. Psychiatry and Clinical Neurosciences, 54(4), 479–485.
Klados, M. A., Kanatsouli, K., Antoniou, I., Babiloni, F., Tsirka, V., Bamidis, P. D., & Micheloyannis, S. (2013). A graph theoretical approach to study the organization of the cortical networks during different mathematical tasks. PLOS ONE, 8(8), e71800. doi:10.1371/journal.pone.0071800
Klimesch, W. (1996). Memory processes, brain oscillations and EEG synchronization. International Journal of Psychophysiology, 24(1), 61–100.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2), 169–195.
LeFevre, J.-A., Bisanz, J., Daley, K. E., Buffone, L., Greenham, S. L., & Sadesky, G. S. (1996). Multiple routes to solution of single-digit multiplication problems. Journal of Experimental Psychology: General, 125(3), 284.
Lemaire, P., & Siegler, R. S. (1995). Four aspects of strategic change: contributions to children’s learning of multiplication. Journal of Experimental Psychology: General, 124(1), 83.
Menon, V. (2010). Developmental cognitive neuroscience of arithmetic: Implications for learning and education. ZDM, 42(6), 515–525.
Menon, V. (2016). Working memory in children’s math learning and its disruption in dyscalculia. Current Opinion in Behavioral Sciences, 10, 125–132.
Menon, V., Rivera, S. M., White, C. D., Glover, G. H., & Reiss, A. L. (2000). Dissociating prefrontal and parietal cortex activation during arithmetic processing. NeuroImage, 12(4), 357–365. doi:10.1006/nimg.2000.0613
Micheloyannis, S., Sakkalis, V., Vourkas, M., Stam, C. J., & Simos, P. G. (2005). Neural networks involved in mathematical thinking: Evidence from linear and non-linear analysis of electroencephalographic activity. Neuroscience Letters, 373(3), 212–217.
Miller, G. A., & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110(1), 40–48.
Mizuhara, H., & Yamaguchi, Y. (2007). Human cortical circuits for central executive function emerge by theta phase synchronization. NeuroImage, 36(1), 232–244.
Moeller, K., Wood, G., Doppelmayr, M., & Nuerk, H.-C. (2010). Oscillatory EEG correlates of an implicit activation of multiplication facts in the number bisection task. Brain Research, 1320, 85–94.
Nemati, P., Schmid, J., Soltanlou, M., Krimly, J.-T., Nuerk, H.-C., & Gawrilow, C. (2017). Planning and self-control, but not working memory, directly predict multiplication performance in adults. Journal of Numerical Cognition (in press).
Neuper, C., & Klimesch, W. (2006). Event-related dynamics of brain oscillations (Vol. 159). New York, NY: Elsevier.
Nunez, P. L., & Cutillo, B. A. (1995). Neocortical dynamics and human EEG rhythms. New York, NY: Oxford University Press.
Obersteiner, A., Dresler, T., Reiss, K., Vogel, A. C. M., Pekrun, R., & Fallgatter, A. J. (2010). Bringing brain imaging to the school to assess arithmetic problem solving: chances and limitations in combining educational and neuroscientific research. ZDM, 42(6), 541–554.
Oostenveld, R., & Praamstra, P. (2001). The five percent electrode system for high-resolution EEG and ERP measurements. Clinical Neurophysiology, 112(4), 713–719.
Petermann, F., Petermann, U., & Wechsler, D. (2007). Hamburg-Wechsler-Intelligenztest für Kinder-IV: (HAWIK-IV). Bern, Switzerland: Huber.
Peters, L., Polspoel, B., de Beeck, H. O., & De Smedt, B. (2016). Brain activity during arithmetic in symbolic and non-symbolic formats in 9–12 year old children. Neuropsychologia, 86, 19–28.
Pfurtscheller, G., & Aranibar, A. (1977). Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalography and clinical neurophysiology, 42(6), 817–826.
Pfurtscheller, G., & Da Silva, F. L. (1999). Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology, 110(11), 1842–1857.
Pfurtscheller, G., Stancak, A., & Neuper, C. (1996). Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology, 24(1), 39–46.
Ploner, N. (2014). RON (ReadOutNumbers) [Computer software]. Germany: Tuebingen.
Polspoel, B., Peters, L., & De Smedt, B. (2016). Strategy over operation: Neural activation in subtraction and multiplication during fact retrieval and procedural strategy use in children. Amsterdam: Paper presented as SIG22 Neuroscience and Education meeting.
Prado, J., Lu, J., Liu, L., Dong, Q., Zhou, X., & Booth, J. R. (2013). The neural bases of the multiplication problem-size effect across countries. Frontiers in Human Neuroscience, 7. doi:10.3389/fnhum.2013.00189
Prado, J., Mutreja, R., & Booth, J. R. (2014). Developmental dissociation in the neural responses to simple multiplication and subtraction problems. Developmental Science, 17(4), 537–552.
Qin, S., Cho, S., Chen, T., Rosenberg-Lee, M., Geary, D. C., & Menon, V. (2014). Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience, 17(9), 1263–1269.
Ranganath, C., Johnson, M. K., & D’Esposito, M. (2003). Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia, 41(3), 378–389.
Rickard, T., Romero, S., Basso, G., Wharton, C., Flitman, S., & Grafman, J. (2000). The calculating brain: An fMRI study. Neuropsychologia, 38(3), 325–335.
Rivera, S. M., Reiss, A., Eckert, M. A., & Menon, V. (2005). Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cerebral Cortex, 15(11), 1779–1790.
Rosenberg-Lee, M., Barth, M., & Menon, V. (2011). What difference does a year of schooling make?: Maturation of brain response and connectivity between 2nd and 3rd grades during arithmetic problem solving. NeuroImage, 57(3), 796–808.
Rosenberg-Lee, M., Lovett, M. C., & Anderson, J. R. (2009). Neural correlates of arithmetic calculation strategies. Cognitive Affective & Behavioral Neuroscience, 9(3), 270–285. doi:10.3758/Cabn.9.3.270
Sammer, G., Blecker, C., Gebhardt, H., Bischoff, M., Stark, R., Morgen, K., & Vaitl, D. (2007). Relationship between regional hemodynamic activity and simultaneously recorded EEG‐theta associated with mental arithmetic‐induced workload. Human Brain Mapping, 28(8), 793–803.
Sankoh, A. J., Huque, M. F., & Dubey, S. D. (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine, 16(22), 2529–2542.
Sauseng, P., & Klimesch, W. (2008). What does phase information of oscillatory brain activity tell us about cognitive processes? Neuroscience & Biobehavioral Reviews, 32(5), 1001–1013.
Siegler, R. S. (1988). Strategy choice procedures and the development of multiplication skill. Journal of Experimental Psychology: General, 117(3), 258.
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V., & Dan, I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage, 27(4), 842–851.
Soltanlou, M., Pixner, S., & Nuerk, H.-C. (2015). Contribution of working memory in multiplication fact network in children may shift from verbal to visuo-spatial: a longitudinal investigation. Frontiers in Psychology, 6. doi:10.3389/fpsyg.2015.01062
Supekar, K., Swigart, A. G., Tenison, C., Jolles, D. D., Rosenberg-Lee, M., Fuchs, L., & Menon, V. (2013). Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proceedings of the National Academy of Sciences, 110(20), 8230–8235.
Sylvester, C.-Y. C., Wager, T. D., Lacey, S. C., Hernandez, L., Nichols, T. E., Smith, E. E., & Jonides, J. (2003). Switching attention and resolving interference: FMRI measures of executive functions. Neuropsychologia, 41(3), 357–370.
Szűcs, D., & Goswami, U. (2007). Educational neuroscience: Defining a new discipline for the study of mental representations. Mind, Brain, and Education, 1(3), 114–127.
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D., & Leahy, R. M. (2011). Brainstorm: A user-friendly application for MEG/EEG analysis. Computational Intelligence and Neuroscience. doi:10.1155/2011/879716
Tschentscher, N., & Hauk, O. (2014). How are things adding up? Neural differences between arithmetic operations are due to general problem solving strategies. NeuroImage, 92, 369–380.
Tsuzuki, D., Jurcak, V., Singh, A. K., Okamoto, M., Watanabe, E., & Dan, I. (2007). Virtual spatial registration of stand-alone fNIRS data to MNI space. NeuroImage, 34(4), 1506–1518.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., … & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289.
Vanbinst, K., & De Smedt, B. (2016). Individual differences in children’s mathematics achievement: The roles of symbolic numerical magnitude processing and domain-general cognitive functions. Progress in Brain Research, 227, 105–130. doi:10.1016/bs.pbr.2016.04.001
Vanbinst, K., Ghesquiere, P., & De Smedt, B. (2014). Arithmetic strategy development and its domain-specific and domain-general cognitive correlates: A longitudinal study in children with persistent mathematical learning difficulties. Research in Developmental Disabilities, 35(11), 3001–3013.
Winer, B. J., Brown, D. R., & Michels, K. M. (1971). Statistical principles in experimental design (Vol. 2). New York, NY: McGraw-Hill.
Witt, M. (2011). School based working memory training: Preliminary finding of improvement in children’s mathematical performance. Advances in Cognitive Psychology, 7, 7–15.
Zago, L., Pesenti, M., Mellet, E., Crivello, F., Mazoyer, B., & Tzourio-Mazoyer, N. (2001). Neural correlates of simple and complex mental calculation. NeuroImage, 13(2), 314–327.
Zamarian, L., Ischebeck, A., & Delazer, M. (2009). Neuroscience of learning arithmetic—Evidence from brain imaging studies. Neuroscience & Biobehavioral Reviews, 33(6), 909–925.
Zhao, H., Tanikawa, Y., Gao, F., Onodera, Y., Sassaroli, A., Tanaka, K., & Yamada, Y. (2002). Maps of optical differential pathlength factor of human adult forehead, somatosensory motor and occipital regions at multi-wavelengths in NIR. Physics in Medicine and Biology, 47(12), 2075–2093.
Zhou, X., Chen, C., Zang, Y., Dong, Q., Chen, C., Qiao, S., & Gong, Q. (2007). Dissociated brain organization for single-digit addition and multiplication. NeuroImage, 35(2), 871–880.
Acknowledgments
We would like to thank all participating children and their parents. This research was funded by a grant from the Science Campus Tuebingen, Project 8.4, to HCN supporting M.S. M.S was also supported by the DFG grant, No. NU 265/3-1 given to H.C.N. C.A., T.D., A.C.E., A.J.F., and H.C.N. are members of the LEAD Graduate School & Research Network [GSC1028], funded by the Excellence Initiative of the German federal and state governments. Furthermore, A.C.E. was partly supported by IZKF Tübingen (Junior Research Group, Grant 2115-0-0). Finally, we thank our assistants who helped with recording EEG and fNIRS data and proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Ann-Christine Ehlis and Hans-Christoph Nuerk equally contributed to this study and should be regarded as joint senior authors.
Rights and permissions
About this article
Cite this article
Soltanlou, M., Artemenko, C., Dresler, T. et al. Increased arithmetic complexity is associated with domain-general but not domain-specific magnitude processing in children: A simultaneous fNIRS-EEG study. Cogn Affect Behav Neurosci 17, 724–736 (2017). https://doi.org/10.3758/s13415-017-0508-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-017-0508-x