
Abstract
Aims: We aimed to assess the efficacy and safety of vitamin K antagonist (VKA) monotherapy in atrial fibrillation (AF) patients undergoing transcatheter aortic valve implantation (TAVI).
Methods and results: In 735 TAVIs since 2008 we identified 167 patients suffering from concomitant AF who received either VKA monotherapy (n=77), VKA plus single antiplatelet therapy (SAPT, n=41) or a triple anticoagulation regimen (n=49). Thromboembolic as well as bleeding complications were analysed for six months after TAVI. Only one minor bleeding and no thromboembolic events occurred after VKA therapy had been initiated post TAVI. Compared to patients being treated with additional either single or dual antiplatelet therapy, the incidence of major/life-threatening bleeding complications was significantly lower in the VKA mono group (0/77 [VKA mono] vs. 3/41 [VKA+SAPT; p=0.04] vs. 4/49 [triple anticoagulation; p=0.02]). Analysis of a combined endpoint of post-procedural death, stroke, embolism and major bleeding revealed a significant superiority of VKA monotherapy compared to VKA plus SAPT or DAPT, respectively (5/77 vs. 9/41 [p=0.02] vs. 14/49 [p=0.002]).
Conclusions: VKA therapy without additional antiplatelet treatment is effective and safe in AF patients undergoing TAVI.
Abbreviations
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
LAAO: left atrial appendage closure
MPG: mean pressure gradient
NOAC: novel oral anticoagulants
OAC: oral anticoagulation
PCI: percutaneous coronary intervention
SAPT: single antiplatelet therapy
SAVR: surgical aortic valve replacement
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
VKA: vitamin K antagonist
Introduction
TAVI has emerged as a valid treatment option in patients with aortic valve stenosis at high or prohibitive risk for surgical aortic valve replacement (SAVR).
The PARTNER trial has confirmed both the superiority of TAVI over medical treatment in inoperable patients and the non-inferiority of TAVI compared with SAVR in high-risk patients1,2. The U.S. CoreValve High Risk Study even showed superiority of TAVI compared to SAVR regarding all-cause mortality3.
After TAVI, dual antiplatelet therapy (DAPT) with lifelong aspirin and clopidogrel for three to six months is currently recommended as standard of care4. However, this practice is not evidence-based (class IIb recommendation, level of evidence C) and is derived in part from experience with percutaneous coronary interventions, similar transcatheter approaches, and knowledge of post-procedure device endothelialisation. Furthermore, this recommendation is largely based on results from an early preliminary first-in-man study reporting two cases of severe thrombocytopaenia following TAVI5. However, there was no definite evidence for a secondary, consumption-mediated mechanism of thrombocytopaenia. Recently, two randomised trials have not shown an additional benefit of clopidogrel to aspirin therapy in reduction of ischaemic and vascular events following TAVI but rather they revealed a trend towards an increased risk of bleeding when employing DAPT6,7.
Patients undergoing TAVI often present with comorbidities (most frequently atrial fibrillation [AF]) necessitating oral anticoagulation (OAC) for the prevention of thromboembolic events. As TAVI patients are susceptible not only to thromboembolic events but also to bleeding complications, this triple anticoagulation regimen is used reluctantly in some centres.
In this study we compared the efficacy and safety of VKA therapy without additional antiplatelet treatment in AF patients undergoing TAVI with VKA plus SAPT and VKA plus DAPT, respectively.
Methods
Between July 2008 and July 2014, 735 transfemoral TAVIs were performed at our institution. During this period, we identified 77 patients suffering from concomitant atrial fibrillation who received VKA therapy without additional antiplatelet treatment, 41 patients who were treated with VKA in combination with SAPT and 49 patients who received a triple anticoagulation regimen after TAVI. As VKA phenprocoumon was used. Patients treated via transapical access were excluded from this analysis. All patients included in our study had symptomatic severe aortic stenosis with an orifice area <1 cm², and were considered as high-risk surgical patients with either a logistic EuroSCORE of ≥20%, an STS score of ≥10% or specific comorbidities that would prohibit surgical therapy, such as porcelain aorta, frailty, chest radiation or severely reduced lung capacity. If possible, all procedures were performed under local anaesthesia and light analgosedation, monitored by a cardiac anaesthesiologist. An activated clotting time of 180-250 s was adjusted by heparin during the procedure. Access-site closure was achieved by applying two ProGlide® SH closure devices (Abbott Vascular, Santa Clara, CA, USA) using the preclosure technique or by applying the Prostar® XL 10 Fr system (Abbott Vascular). Patients were transferred to our intensive care unit after the procedure (for at least 24 hrs). VKA therapy was usually initiated one week after TAVI. Within the first post-procedural days, the start and dosage of overlapping heparinisation were decided on an individual basis. Most patients were discharged within two weeks. Follow-up visits in our outpatient clinic were scheduled four weeks and six months after TAVI. Patients not keeping the appointment were contacted by phone, if applicable. Thromboembolic as well as bleeding complications were analysed according to VARC-2 criteria8 for a period of six months after TAVI.
All patients were informed about specific risks and alternatives of TAVI and gave written informed consent to TAVI and pre- and post-interventional monitoring (data collection). The study protocol was in accordance with the local ethics committee.
STATISTICAL ANALYSIS
The Kolmogorov-Smirnov equation was used to test for normal distribution. Continuous variables with normal distribution were compared between groups using the Student’s t-test with mean±1 standard deviation reported. For continuous variables without normal distribution a Mann-Whitney U test was performed. Fisher’s exact test was applied to compare categorical variables with frequency and percent reported. A log-rank test was performed for comparison of Kaplan-Meier curves. A p-value of less than 0.05 was considered statistically significant. For statistical analysis, GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) as well as MedCalc® software (MedCalc Software bvba, Ostend, Belgium) was used.
Results
PATIENT CHARACTERISTICS
There were no significant differences in baseline characteristics comparing the VKA mono group with the other groups. In particular, CHA2DS2-VASc score (4.9±1.1 vs. 4.9±1.5 [p=0.82]; 4.9±1.1 vs. 4.7±1.3 [p=0.32])9, HEMORR2HAGES score (5.1±1.9 vs. 5.0±1.5 [p=0.74]; 5.1±1.9 vs. 5.2±1.8 [p=0.71])10 and HAS-BLED score (2.9±0.9 vs. 2.9±0.9 [p=0.21]; 2.9±0.9 vs. 2.9±1.0 [p=0.64])11 did not differ significantly between the VKA mono group vs. VKA+SAPT or the triple anticoagulation group, respectively. Comorbidities, such as previous cardiovascular surgery and prior stroke or TIA, were equally distributed among the groups as was the frequency of chronic renal and heart failure. Pre-interventional thrombocyte count and haemoglobin levels did not differ significantly (Table 1).
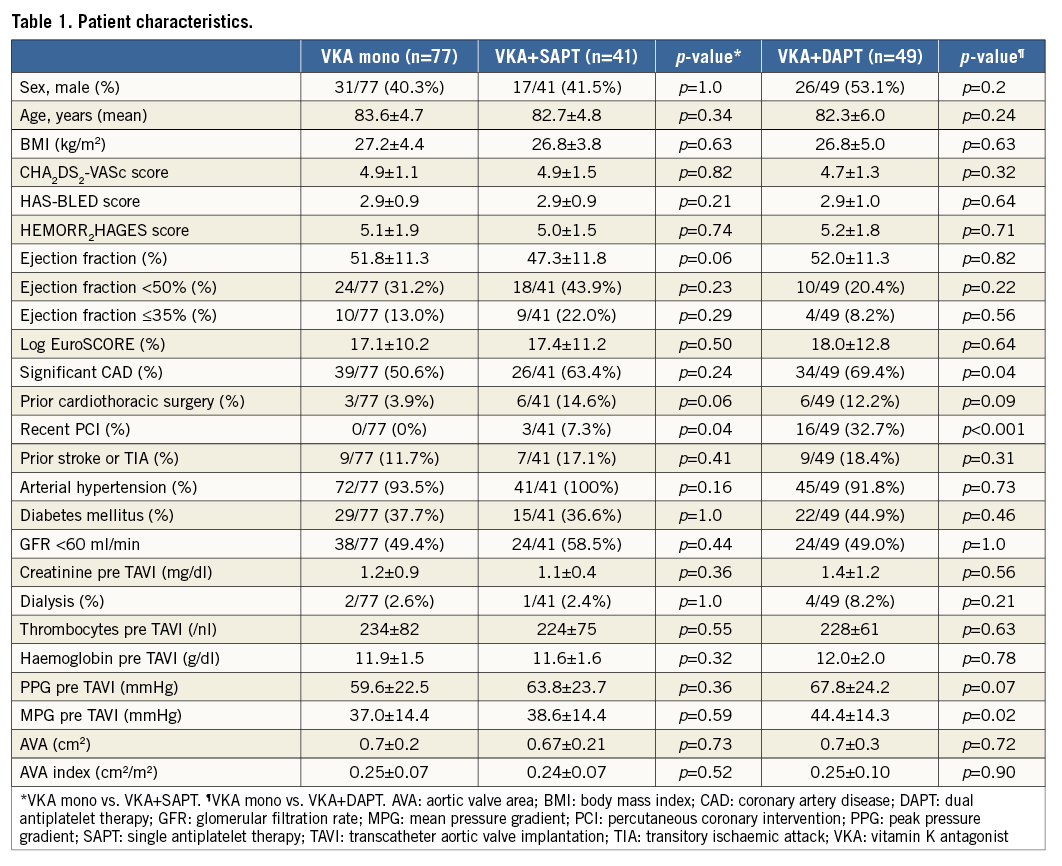
Only mean pressure gradient (MPG) showed a significant but clinically irrelevant difference between the VKA mono and triple anticoagulation groups (37.0±14.4 mmHg vs. 44.4±14.3 mmHg; p<0.05) as did coronary artery disease (CAD) (39/77, 50.6% vs. 34/49, 69.4%; p<0.05). The latter is attributable to a large proportion of patients in the triple anticoagulation group who received a coronary stent implantation in a relevant time frame prior to TAVI (16/49, 32.7% vs. 0/77; 0%; p<0.001), thus even more significantly necessitating dual antiplatelet treatment in addition to OAC. Concordantly, prior coronary stent implantation represented the most frequent reason for a triple anticoagulation regimen post TAVI.
A detailed overview of patients’ demographic characteristics is shown in Table 1.
PROCEDURAL CHARACTERISTICS
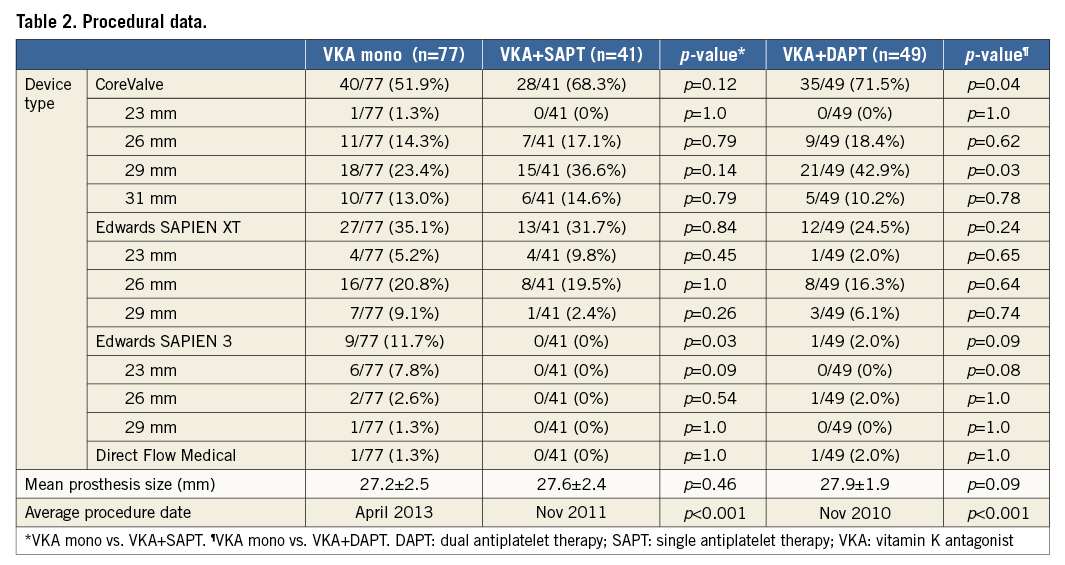
In the VKA mono group, 40 CoreValve® (Medtronic, Minneapolis, MN, USA), 27 Edwards SAPIEN XT, nine Edwards SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) and one Direct Flow Medical® (Direct Flow Medical Inc., Santa Rosa, CA, USA) prostheses were implanted. In the VKA plus SAPT group, 28 CoreValve and 13 Edwards SAPIEN XT prostheses were used. In the triple anticoagulation group, 35 CoreValve, 12 Edwards SAPIEN XT, one Edwards SAPIEN 3 and one Direct Flow Medical prostheses were implanted. Mean prosthesis size was 27.2±2.5 mm (VKA mono), 27.6±2.4 mm (VKA+SAPT) and 27.9±1.9 mm (triple anticoagulation) (Table 2).
FOLLOW-UP
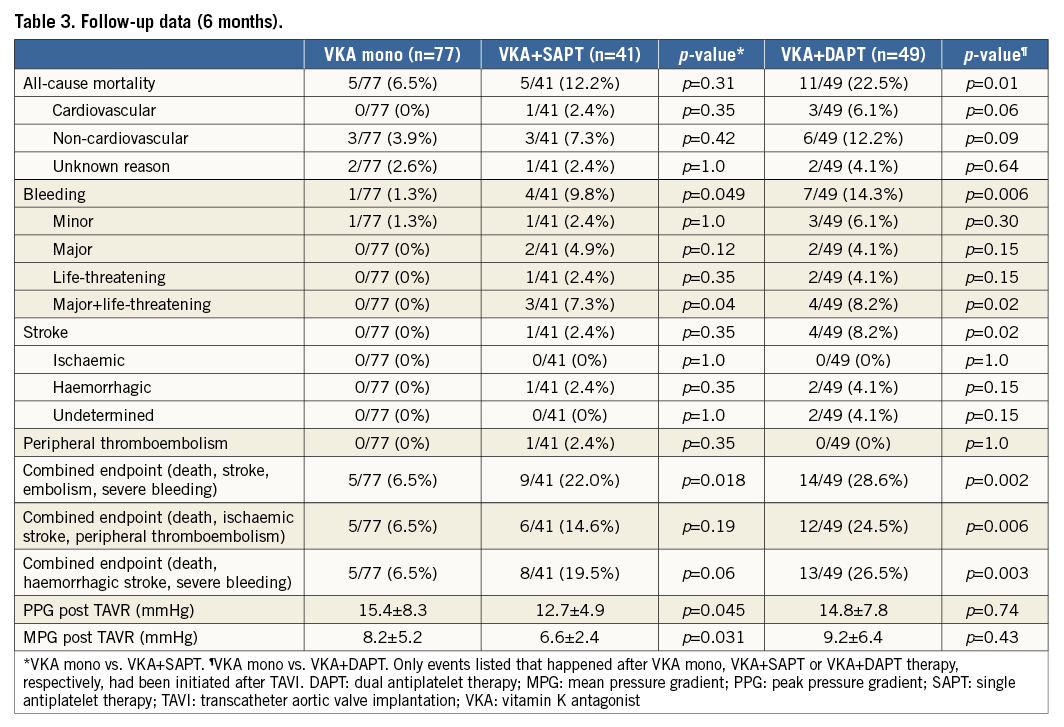
In 165/167 (98.8%) patients, six-month follow-up information was available (Table 3).
MORTALITY
In the VKA mono group, five patients (6.5%) died within six months after TAVI (three non-cardiovascular/no bleeding, two unknown reasons). This is significantly less (p=0.01) compared to the all-cause mortality rate in the triple anticoagulation group (11 patients [22.5%] died during the relevant follow-up period [six non-cardiovascular, three cardiovascular, two unknown reasons]). In the VKA plus SAPT group, five patients (12.2%) died (three non-cardiovascular, one cardiovascular, one unknown reasons) (Figure 1).
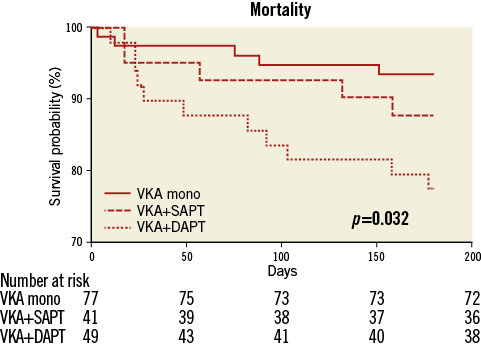
Figure 1. Kaplan-Meier curves for survival in the first six months after TAVI.
BLEEDING EVENTS
In the VKA mono group, bleeding complications occurred in five patients (6.5%). Only in one of those patients (1.3%, gastrointestinal bleeding due to a polyp four months after TAVI) had VKA therapy already been initiated after the intervention. The other haemorrhages occurred peri-interventionally (one pericardial effusion and one bleeding from the puncture site) or within seven days after TAVI (two haematurias due to urinary catheters), thus not related to anticoagulation therapy with phenprocoumon. In the triple anticoagulation group, bleeding complications were observed in ten patients (20.4%). Three of those haemorrhages (one pericardial effusion, two haematurias due to urinary catheters) occurred peri-interventionally or within seven days after TAVI, respectively. In those patients, triple anticoagulation had not been initiated at the time of the bleeding event. In patients effectively being treated with OAC in combination with dual antiplatelet therapy, bleeding complications were observed in seven cases (14.3%) within six months after TAVI: two fatal intracranial haemorrhages occurred two and three months after TAVI, respectively. Moreover, two major bleedings (one thigh haematoma requiring a two-stage operation, one gastrointestinal bleeding from a duodenal ulcer) and three minor bleedings (one recurrent haemoptysis, one recurrent epistaxis, one macrohaematuria) were observed within six months post procedure. In the VKA plus SAPT group, eight bleeding events (19.5%) were observed. Four of those haemorrhages (one retroperitoneal haematoma, one haematoma at the puncture site requiring surgery, one haematuria due to a urinary catheter, one lower gastrointestinal bleeding) occurred peri-interventionally or within seven days after TAVI, respectively. In patients effectively being treated with OAC in combination with single antiplatelet therapy, bleeding complications were observed in four cases (9.8%) within six months after TAVI: one life-threatening (intracranial haemorrhage) bleeding, two major (one upper gastrointestinal bleeding, one recurrent epistaxis requiring tamponade) bleeding and one minor bleeding (recurrent epistaxis) occurred within six months post TAVI. Thus, compared to the VKA mono group, the incidence of overall bleeding events after initiation of antithrombotic therapy (1/77 vs. 4/41 [p=0.049] vs. 7/49 [p=0.006]), as well as the incidence of severe bleeding events (life-threatening and major), was significantly higher in the VKA plus SAPT group as well as in the triple anticoagulation group (0/77 vs. 3/41 [p=0.04] vs. 4/49 [p=0.02]) (Figure 2).
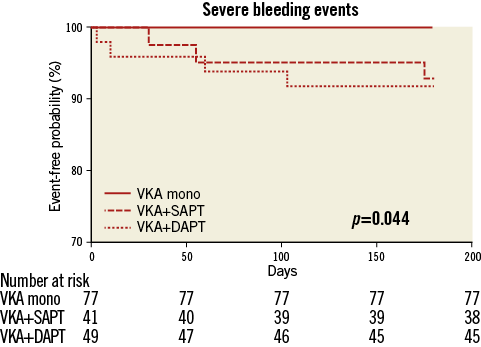
Figure 2. Kaplan-Meier curves for severe bleeding events (life-threatening and major) after initiation of antithrombotic therapy in the first six months after TAVI.
Analysis of a composite endpoint of post-procedural (≥72 hrs after TAVI) death, haemorrhagic stroke and severe bleeding revealed a significant superiority of VKA monotherapy compared to a triple anticoagulation regimen (5/77 vs. 13/49 [p=0.003] events) and a trend towards superiority compared to VKA plus SAPT (5/77 vs. 8/41 [p=0.06] events) (Figure 3).
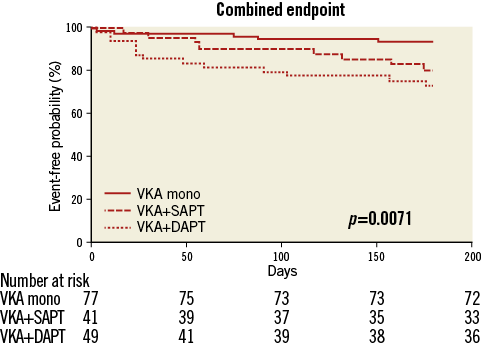
Figure 3. Kaplan-Meier curves for a composite endpoint of post-procedural (≥72 hrs after TAVI) death, haemorrhagic stroke and severe bleeding in the first six months after TAVI.
THROMBOEMBOLIC EVENTS
In the VKA mono group, no thromboembolic events occurred after OAC had been initiated. Only one patient (1.3%) experienced a transitory ischaemic attack on the first day after TAVI. In the VKA plus SAPT group, one peripheral embolism (ischaemic optic neuropathy; 2.4%) and one (haemorrhagic) stroke (2.4%) occurred within six months after TAVI (both ≥72 hrs after TAVI). In the triple anticoagulation group, five strokes (two haemorrhagic, one ischaemic, two undetermined; 10.2%) were observed (one <72 hrs and four ≥72 hrs after TAVI). Thus, the incidence of post-procedural strokes (≥72 hrs after TAVI) was significantly higher in the triple anticoagulation group compared to the VKA mono group (p=0.02).
During the follow-up period of six months, no newly developed intracardiac thrombi could be detected in the course of follow-up transthoracic and transoesophageal echocardiograms. Furthermore, transvalvular gradients did not indicate thrombosis of the transcatheter prostheses, with a statistically significant but clinically irrelevant difference between the VKA mono and VKA plus SAPT group (VKA mono group: peak pressure gradient [PPG] 15.4±8.3 mmHg, mean pressure gradient [MPG] 8.2±5.2 mmHg; VKA plus SAPT group: PPG 12.7±4.9 mmHg [p<0.05], MPG 6.6±2.4 mmHg [p<0.05]; triple anticoagulation group: PPG 14.8±7.8 mmHg, MPG 9.2±6.4 mmHg).
Analysis of a composite endpoint of post-procedural (≥72 hrs after TAVI) death, ischaemic stroke and peripheral thromboembolism revealed a significant superiority of VKA monotherapy compared to a triple anticoagulation regimen (5/77 vs. 12/49 [p=0.006] events). No significant differences were observed comparing VKA monotherapy to VKA plus SAPT (5/77 vs. 6/41 [p=0.19] events) (Figure 4).
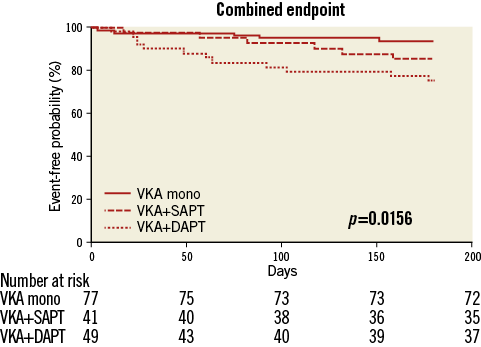
Figure 4. Kaplan-Meier curves for a composite endpoint of post-procedural (≥72 hrs after TAVI) death, ischaemic stroke and peripheral thromboembolism in the first six months after TAVI.
COMBINED ENDPOINT
Analysis of a combined endpoint of post-procedural (≥72 hrs after TAVI) death, stroke, embolism and severe bleeding revealed a significant superiority of VKA monotherapy compared to VKA plus SAPT or a triple anticoagulation regimen, respectively (5/77 vs. 9/41 [p=0.02] vs. 14/49 [p=0.002] events) (Figure 5).
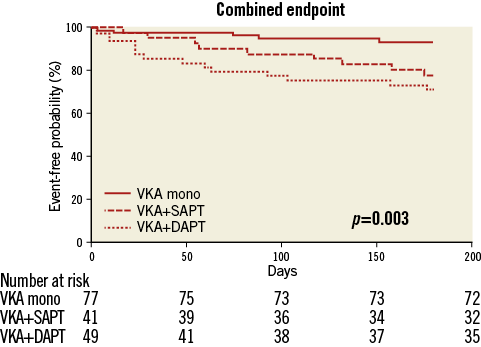
Figure 5. Kaplan-Meier curves for a combined endpoint of post-procedural (≥72 hrs after TAVI) death, stroke, embolism and severe bleeding in the first six months after TAVI.
Discussion
ANTITHROMBOTIC THERAPY AFTER TAVI
After TAVI, the most widely used antithrombotic strategy is DAPT with lifelong aspirin in combination with clopidogrel for three to six months12-14. However, this practice has never been validated in clinical trials and there is no robust evidence demonstrating that early thromboembolic events observed after TAVI are platelet-mediated. Moreover, current study data have not revealed an additional benefit of clopidogrel to aspirin therapy in terms of the reduction of ischaemic and vascular events following TAVI but rather have shown a trend towards an increased bleeding risk when applying DAPT6,7,15. However, given the limited number of patients studied so far, these findings need to be confirmed in a larger trial. To that end, the ongoing ARTE trial (aspirin versus aspirin+clopidogrel following transcatheter aortic valve implantation) continues to compare the hypothesis of single antiplatelet therapy versus DAPT after TAVI in patients not requiring systemic anticoagulation (NCT01559298).
Oral anticoagulation has also been considered as part of the antithrombotic strategy post TAVI, since there is still an imperfect understanding of the nature of thromboembolic events post TAVI, and whether they are primarily platelet-based or thrombin-based. Currently, the Dual Antiplatelet Therapy Versus Oral Anticoagulation for a Short Time to Prevent Cerebral Embolism After TAVI (AUREA) trial is ongoing to examine DAPT versus OAC (employing Sintrom-acenocoumarol) in the prevention of cerebral thromboembolism (NCT01642134).
ANTITHROMBOTIC THERAPY AFTER SURGICAL AORTIC VALVE REPLACEMENT USING A BIOPROSTHESIS
The main advantage of bioprostheses is that long-term anticoagulant therapy is not mandatory, unless there are other indications for OAC16. In these cases, indications and target INR conform to the recommendations concerning the associated disease, most often AF. The need for postoperative anticoagulant therapy has been challenged for patients receiving an aortic bioprosthesis without concomitant indications for OAC since the thromboembolic risk may not justify the bleeding risk of anticoagulant therapy. However, scientific data available on this topic are limited and contradictory. In a large-scale retrospective analysis from an observational registry of the STS adult cardiac surgery database, no significant difference between aspirin monotherapy and VKA monotherapy was found. However, the combination of VKA and aspirin was associated with a decrease in thromboembolic events at the expense of an increase in bleeding, with a lower adjusted risk of death17. Another analysis of a large-scale surgical Danish database showed a higher incidence of thromboembolic events and of cardiovascular mortality in patients discontinuing warfarin during the first six postoperative months. Interestingly, there was no excess bleeding in warfarin-treated patients compared to a heterogeneous group of patients either having any antithrombotic therapy or receiving aspirin mono treatment after discontinuation of VKA18. No data on treatment with VKA plus DAPT after implantation of a surgical aortic bioprosthesis exist.
In a synopsis of the available data, ESC/EACTS and AHA/ACC guidelines as well as the Ninth ACCP consensus consistently favour the use of aspirin alone vs. VKA during the postoperative period after aortic valve replacement using a bioprosthesis16.
TAVI PATIENTS WITH CONCOMITANT INDICATIONS FOR OAC
Patients who undergo TAVI frequently suffer from comorbidities representing concomitant indications for anticoagulation, such as atrial fibrillation. AF is observed in ~30% of patients undergoing TAVI and is associated with a greater than twofold increased risk of all-cause and cardiovascular mortality, irrespective of the type of AF19. In those patients, a high degree of variability exists regarding antithrombotic therapy, ranging from VKA alone to triple therapy applying OAC in combination with DAPT. As TAVI patients are susceptible not only to thromboembolic events but also to bleeding complications, careful risk/benefit stratification on an individual basis is necessary to determine optimum antithrombotic therapy. Recent investigations comparing triple therapy and DAPT following myocardial infarction or PCI in AF patients showed a significant reduction in the rate of major bleedings (HR 0.48, 95% CI: 0.38-0.61) at the cost of an increase in the rate of ischaemic strokes (HR 1.50, 95% CI: 1.03-2.20) when applying DAPT20.
This finding is consistent in all PCI trials comparing triple therapy versus DAPT21-23. In patients undergoing coronary interventions, according to the results of a National Danish Registry and the WOEST (What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting?) trial, the combination of OAC and clopidogrel appears attractive and was not associated with an excess of myocardial infarction or coronary death as compared to triple therapy but instead displayed a better safety profile20,21.
CURRENT CONSENSUS GUIDELINES
Given this experience, largely derived from PCI trials, the current consensus guidelines suggest that, in patients with concomitant AF undergoing TAVI, the decision can be made partially based on the strength of indication for DAPT, for example recent PCI. Thus, in AF patients with recent PCI requiring DAPT who undergo TAVI, it can be reasonable to pursue triple therapy. In patients with indications for OAC, in the absence of a strong indication for DAPT post TAVI, it is reasonable to pursue single antiplatelet therapy with anticoagulation4.
However, these strategies have never been evaluated prospectively in post-TAVI procedures.
VKA MONOTHERAPY IN AF PATIENTS UNDERGOING TAVI
Our data suggest that VKA therapy without additional antiplatelet treatment is effective and safe in AF patients undergoing TAVI. Within the relevant follow-up period of six months, only one minor bleeding occurred after VKA therapy had been re-initiated after TAVI.
Compared to patients being treated with additional either single or dual antiplatelet therapy, significantly fewer bleeding complications were observed when applying VKA monotherapy. Importantly, bleeding events in patients receiving additional antiplatelet treatment were predominantly severe, including three intracranial haemorrhages.
Furthermore, the incidence of post-procedural strokes was significantly higher in the triple anticoagulation group. In addition, omitting concomitant antiplatelet treatment did not result in an increased rate of overall thromboembolic events and neither newly developed intracardiac thrombi nor transcatheter heart valve thrombosis developed applying VKA monotherapy.
Moreover, all-cause mortality was significantly higher in the triple anticoagulation group, and analysing a combined endpoint of post-procedural death, stroke, embolism and severe bleeding revealed a significant superiority of VKA monotherapy compared to VKA plus SAPT or a triple anticoagulation regimen.
Thus, VKA monotherapy seems to be a valid treatment option for patients undergoing TAVI and suffering from comorbidities necessitating OAC. Whenever applicable, triple anticoagulation therapy should be avoided and always restricted to a minimum if mandatory.
LEFT ATRIAL APPENDAGE CLOSURE
Another approach, reasonable for high-risk AF patients undergoing TAVI and necessitating additional DAPT, is a transcatheter left atrial appendage closure (LAAO), a newly accepted therapeutic option in selected AF patients who have contraindications for an OAC therapy and a high cardioembolic risk. Successful LAAO following TAVI has been described in a case report by Bogunovic et al24.
Study limitations
This study is limited by the retrospective data analysis, and prospective trials are certainly needed. Moreover, the single-centre observational design and the lack of an external core lab adjudicating the events limit data interpretation.
In addition, novel oral anticoagulants (NOAC) have not been analysed here. With the increased use of NOAC, this novel therapeutic concept in combination with TAVI will have to be studied. However, these data are helpful in developing the confidence of using NOAC monotherapy in TAVI patients with AF in the future.
Conclusions
The optimal management of antithrombotic strategies in various patient populations following TAVI remains an evolving field, and further large-scale, randomised studies are necessary to define optimum antithrombotic therapy, especially in patients with concomitant indications for systemic anticoagulation. The results of our study suggest that VKA therapy without additional antiplatelet treatment is effective and safe in atrial fibrillation patients undergoing transcatheter aortic valve implantation.
| Impact on daily practice After transcatheter aortic valve implantation, the prescription of dual antiplatelet therapy for three to six months is widely accepted. However, this practice is not evidence-based and, in patients with concomitant indications for oral anticoagulation, a high degree of variability exists regarding antithrombotic therapy, ranging from a vitamin K antagonist (VKA) alone to triple therapy. The results of our study suggest that VKA therapy without additional antiplatelet treatment is effective and safe in atrial fibrillation patients undergoing transcatheter aortic valve implantation. |
Conflict of interest statement
N. Geis, R. Bekeredjian and E. Chorianopoulos are investigators in the ADVANCE II and SIMPLIFy TAVI trials. The other authors have no conflicts of interest to declare.




