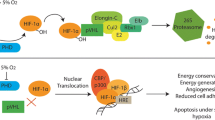
Abstract
Oxygen is an essential nutrient for cells. Oxygen is delivered to tissues via red blood cells through the vasculature. Molecular mechanisms mediating cellular responses to low oxygen tension have been identified. Hypoxia-inducible factors (HIFs) are activated by low oxygen and promote transcriptional regulation of downstream effector genes, which lead to cellular adaptations. Controlled hypoxia exposure is utilized as an experimental tool to investigate biological processes, regulating cellular adaptations. Here we describe detailed protocols for hypoxia exposure of pregnant rodent models and low oxygen exposure of trophoblast stem cells, utilizing gas-regulated chamber systems. The presentation also includes phenotypic analyses of the manipulated animal models and cells.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93(4):266–276
Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9(4):285–296
Rodesch F, Simon P, Donner C et al (1992) Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 80(2):283–285
Semenza GL (2010) Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2(3):336–361
Adelman DM, Gertsenstein M, Nagy A et al (2000) Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14(24):3191–3203
Alsat E, Wyplosz P, Malassine A et al (1996) Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol 168(2):346–353
Caniggia I, Mostachfi H, Winter J et al (2000) Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 105(5):577–587
Genbacev O, Joslin R, Damsky CH et al (1996) Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97(2):540–550
James JL, Stone PR, Chamley LW (2006) The effects of oxygen concentration and gestational age on extravillous trophoblast outgrowth in a human first trimester villous explant model. Hum Reprod 21(10):2699–2705
Jiang B, Kamat A, Mendelson CR (2000) Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol 14(10):1661–1673
Nelson DM, Johnson RD, Smith SD et al (1999) Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol 180(4):896–902
Burton GJ (2009) Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215(1):27–35
Burton GJ, Jaunaiux E (2001) Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynecol Obstet Fertil 29(7–8):503–508
Fryer BH, Simon MC (2006) Hypoxia, HIF and the placenta. Cell Cycle 5(5):495–498
Cowden Dahl KD, Fryer BH et al (2005) Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol 25(23):10479–10491
Gnarra JR, Ward JM, Porter FD et al (1997) Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A 94(17):9102–9107
Maltepe E, Krampitz GW, Okazaki KM et al (2005) Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 132(15):3393–3403
Takeda K, Ho VC, Takeda H et al (2006) Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26(22):8336–8346
Rosario GX, Konno T, Soares MJ (2008) Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 314(2):362–375
Chakraborty D, Cui W, Rosario GX et al (2016) HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A 113(46):E7212–E7221
Chakraborty D, Rumi MA, Konno T et al (2011) Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci U S A 108(39):16295–16300
Chakraborty D, Rumi MA, Soares MJ (2012) NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle 11(13):2427–2430
Asanoma K, Rumi MA, Kent LN et al (2011) FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol 351(1):110–119
Acknowledgments
We acknowledge present and past members of the Soares Laboratory for their contributions to the development of these research model systems. This work was supported by NIH HD020676.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Chakraborty, D., Scott, R.L., Soares, M.J. (2018). Hypoxia Signaling and Placental Adaptations. In: Huang, L. (eds) Hypoxia. Methods in Molecular Biology, vol 1742. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7665-2_15
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7665-2_15
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7664-5
Online ISBN: 978-1-4939-7665-2
eBook Packages: Springer Protocols